


Navigating the complex landscape of clinical trials requires strict adherence to regulatory requirements, especially regarding Trial Master File (TMF) reconciliation for inspections by BDA. Organizations that excel in this area not only ensure compliance but also significantly enhance the quality and efficiency of their studies. Yet, with evolving guidelines and increasing scrutiny, many organizations encounter substantial challenges in managing their TMF effectively.
What strategies can organizations adopt to elevate their TMF practices and prepare for the future of clinical research? By addressing these challenges head-on, they can position themselves for success in an ever-changing environment. The importance of mastering TMF management cannot be overstated, as it serves as a cornerstone for compliance and operational excellence in clinical research.
Navigating the complex regulatory environment governing clinical studies requires effective TMF reconciliation for inspections by BDA. Understanding the guidelines from the FDA, EMA, and ICH is not just beneficial; it’s crucial. Regulations require complete and accurate documentation, timely updates, and the capability for TMF reconciliation for inspections by BDA to demonstrate compliance. Recent statistics reveal that many organizations still struggle with TMF reconciliation for inspections by BDA, as inspection findings consistently highlight gaps in archiving practices and retention policies.
The introduction of the ICH E6(R3) guidelines in early 2025 underscores the necessity of maintaining a comprehensive TMF that accurately reflects study conduct and data integrity. By proactively reviewing and adapting to these evolving regulations, organizations can significantly enhance their TMF practices. This not only ensures compliance but also prepares them for TMF reconciliation for inspections by BDA, fostering a culture of regulatory adherence.
Moreover, aligning TMF practices with these guidelines enhances the overall quality and efficiency of clinical studies. As the landscape of clinical research continues to evolve, organizations must recognize the importance of collaboration and the next steps in refining their processes. Are you ready to take action and elevate your TMF practices?

Establishing systematic processes for Trial Master File (TMF) management is essential for ensuring accuracy and compliance in clinical trials. With regulatory scrutiny set to intensify as the new framework takes effect in April 2026, organizations must prioritize clear workflows that delineate each step of the TMF reconciliation for inspections by BDA, from creation to final archiving. This involves:
For instance, conducting regular completeness evaluations can swiftly identify missing files or inconsistencies early in the process. This is particularly critical, given that recent inspections revealed that 12 out of 30 entities faced issues related to discrepancies. Moreover, employing tailored checklists for specific studies not only enhances consistency but also ensures that all necessary documentation is accounted for.
The adoption of electronic Trial Master Files (eTMFs) can further streamline workflows, providing instant access to documents and simplifying TMF management. By embracing a systematic approach and consistently updating the TMF Plan throughout the study, organizations can significantly minimize errors and optimize the TMF reconciliation for inspections by BDA. This proactive strategy facilitates smoother preparations for inspections and ensures compliance with evolving regulatory standards.

Effective TMF reconciliation for inspections by BDA is crucial for success in clinical research, relying heavily on robust collaboration and communication among team members. Statistics reveal that 68% of employees struggle with the pace and volume of work, while 46% report feelings of burnout. These factors can significantly hinder effective communication within clinical trial teams. To address these challenges, organizations must foster open dialogue and conduct regular check-ins, ensuring that all stakeholders are well-informed about TMF status and any emerging issues.
Utilizing collaborative tools, such as shared digital platforms, allows for real-time updates and document sharing, streamlining teamwork. For instance, entities that adopt structured communication strategies report improved engagement and efficiency; teams using Slack send 32% fewer internal emails, showcasing the effectiveness of digital platforms for TMF communication. Establishing a culture of transparency encourages team members to discuss challenges openly, leading to quicker resolutions. As Andrew Carnegie wisely noted, 'Teamwork is the ability to work together toward a common vision… It is the fuel that allows common people to attain uncommon results.'
Regular cross-functional meetings can further identify potential bottlenecks, enabling teams to collaboratively strategize solutions. By nurturing a collaborative environment, organizations can significantly enhance the effectiveness of their TMF reconciliation for inspections by BDA.

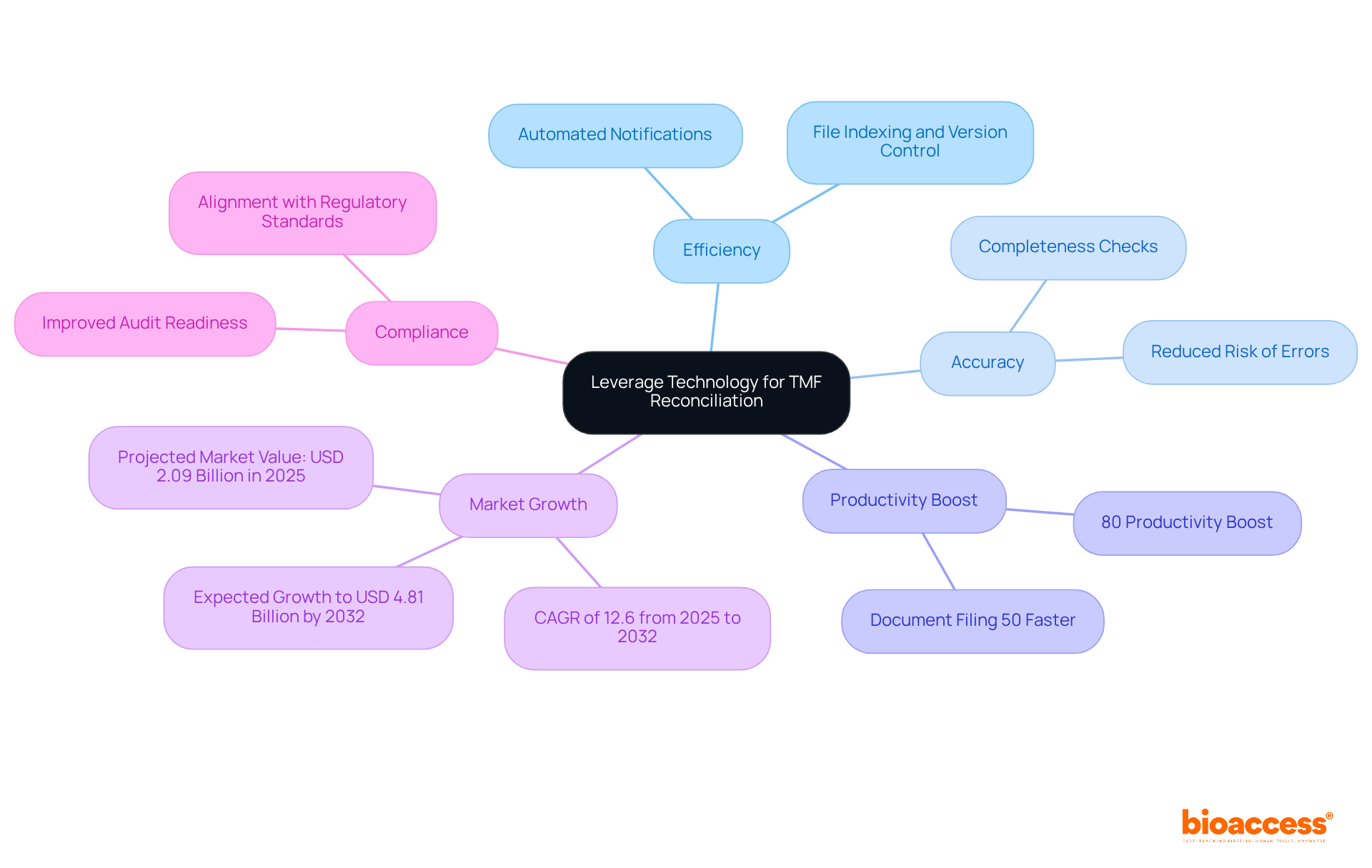
Integrating technology into TMF reconciliation processes can significantly enhance both efficiency and accuracy in clinical research. Organizations are increasingly adopting electronic Trial Master File (eTMF) systems, which facilitate real-time management and tracking of files. These systems automate routine tasks like file indexing, version control, and completeness checks, thereby reducing the risk of errors and allowing team members to concentrate on more strategic initiatives. For instance, automated notifications for missing files can proactively address issues before they escalate, ultimately improving compliance.
Moreover, leveraging data analytics tools offers valuable insights into TMF health metrics, enabling organizations to pinpoint trends and identify areas for improvement. A survey conducted by Veeva Systems revealed that 80% of clinical study sponsors utilizing eTMF reported a productivity boost, with document filing completed up to 50% faster than traditional methods. The eTMF market is projected to reach a valuation of USD 2.09 billion in 2025, with expectations to grow to USD 4.81 billion by 2032, highlighting substantial growth potential.
By harnessing technology, organizations can streamline their TMF reconciliation for inspections by BDA, ensuring compliance and readiness. This strategic approach not only leads to more efficient clinical trials but also positions organizations to tackle the evolving challenges in the Medtech landscape.
Navigating the complex landscape of Trial Master File (TMF) reconciliation is crucial for ensuring compliance and achieving success in clinical research. Understanding regulatory requirements, implementing systematic processes, fostering team collaboration, and leveraging technology are essential for enhancing TMF reconciliation, especially in preparation for inspections by BDA. By adopting these best practices, organizations can not only meet regulatory expectations but also significantly improve the quality and efficiency of their clinical trials.
Key arguments highlight the necessity of:
The integration of technology, particularly electronic Trial Master Files (eTMFs), plays a vital role in streamlining processes and ensuring timely updates. This ultimately leads to better compliance and readiness for inspections.
As the regulatory environment continues to evolve, embracing these best practices is not just advisable; it is imperative for organizations aiming to excel in clinical trials. By prioritizing TMF reconciliation and cultivating a culture of collaboration and innovation, stakeholders can enhance their operational effectiveness and contribute to the advancement of clinical research. Taking proactive steps today will pave the way for a more compliant and efficient future in TMF management.
What is TMF reconciliation and why is it important for clinical studies?
TMF reconciliation is the process of ensuring that the Trial Master File (TMF) is complete, accurate, and up-to-date, which is crucial for inspections by regulatory bodies like the BDA. It demonstrates compliance with regulatory requirements.
Which regulatory bodies provide guidelines for TMF reconciliation?
The guidelines for TMF reconciliation are provided by the FDA (Food and Drug Administration), EMA (European Medicines Agency), and ICH (International Council for Harmonisation).
What are the key requirements for TMF documentation according to regulations?
Regulations require that TMF documentation is complete, accurate, updated in a timely manner, and capable of being reconciled for inspections by the BDA.
What challenges do organizations face regarding TMF reconciliation?
Many organizations struggle with TMF reconciliation, as inspection findings often reveal gaps in archiving practices and retention policies.
What is the significance of the ICH E6(R3) guidelines introduced in early 2025?
The ICH E6(R3) guidelines emphasize the need for a comprehensive TMF that accurately reflects study conduct and data integrity, highlighting the importance of adapting to evolving regulations.
How can organizations improve their TMF practices?
Organizations can enhance their TMF practices by proactively reviewing and adapting to the latest regulations, which not only ensures compliance but also improves the quality and efficiency of clinical studies.
What role does collaboration play in refining TMF processes?
Collaboration is essential in refining TMF processes as it helps organizations navigate the evolving landscape of clinical research and fosters a culture of regulatory adherence.