


Understanding the complexities of biomedical research requires a thorough examination of the methodologies employed in the field. In vitro and in vivo research approaches each present distinct advantages and limitations, shaping how scientists investigate biological processes and develop new treatments. As researchers navigate the intricate landscape of drug discovery and safety testing, a critical question arises: how do these methodologies complement one another? What factors should guide the choice between them? Exploring this dynamic interplay not only illuminates the efficacy of scientific inquiry but also underscores the ethical considerations that underpin responsible research practices.
Laboratory research plays a crucial role in clinical studies, encompassing investigations conducted outside living organisms, typically within controlled environments like petri dishes or test tubes. The term 'in glass,' derived from Latin, underscores the artificial nature of these experiments. This methodology allows researchers to isolate specific biological components, such as cells or tissues, enabling a detailed analysis of cellular behavior and interactions under tightly controlled conditions. In vitro experiments are particularly advantageous for early-stage drug screening and mechanistic investigations, yielding faster results and reducing reliance on animal testing.
Conversely, experiments conducted within living organisms provide a comprehensive understanding of biological processes. The phrase 'in the living,' which translates to 'within the living,' indicates that these studies occur in the organism's natural biological context. In vivo methodologies are essential for grasping the complex interactions within tissues and organs, as they account for the physiological environment that influences biological responses. Current trends indicate a growing integration of advanced technologies, such as organ-on-chip systems, to enhance the precision and ethical standards of in situ studies.
In biomedical research, both in vitro and invivo methodologies are indispensable, each offering unique insights and advantages. In vivo research is critical for assessing drug effectiveness, safety, and pharmacokinetics, while in vitro and invivo experiments excel in providing targeted insights into cellular mechanisms. Understanding the strengths and limitations of each approach is vital for researchers to make informed decisions that align with their study objectives. Katherine Ruiz, a regulatory affairs specialist with experience at INVIMA, emphasizes the importance of adhering to regulations for medical devices and diagnostic tests, ensuring that innovations meet safety and efficacy standards.
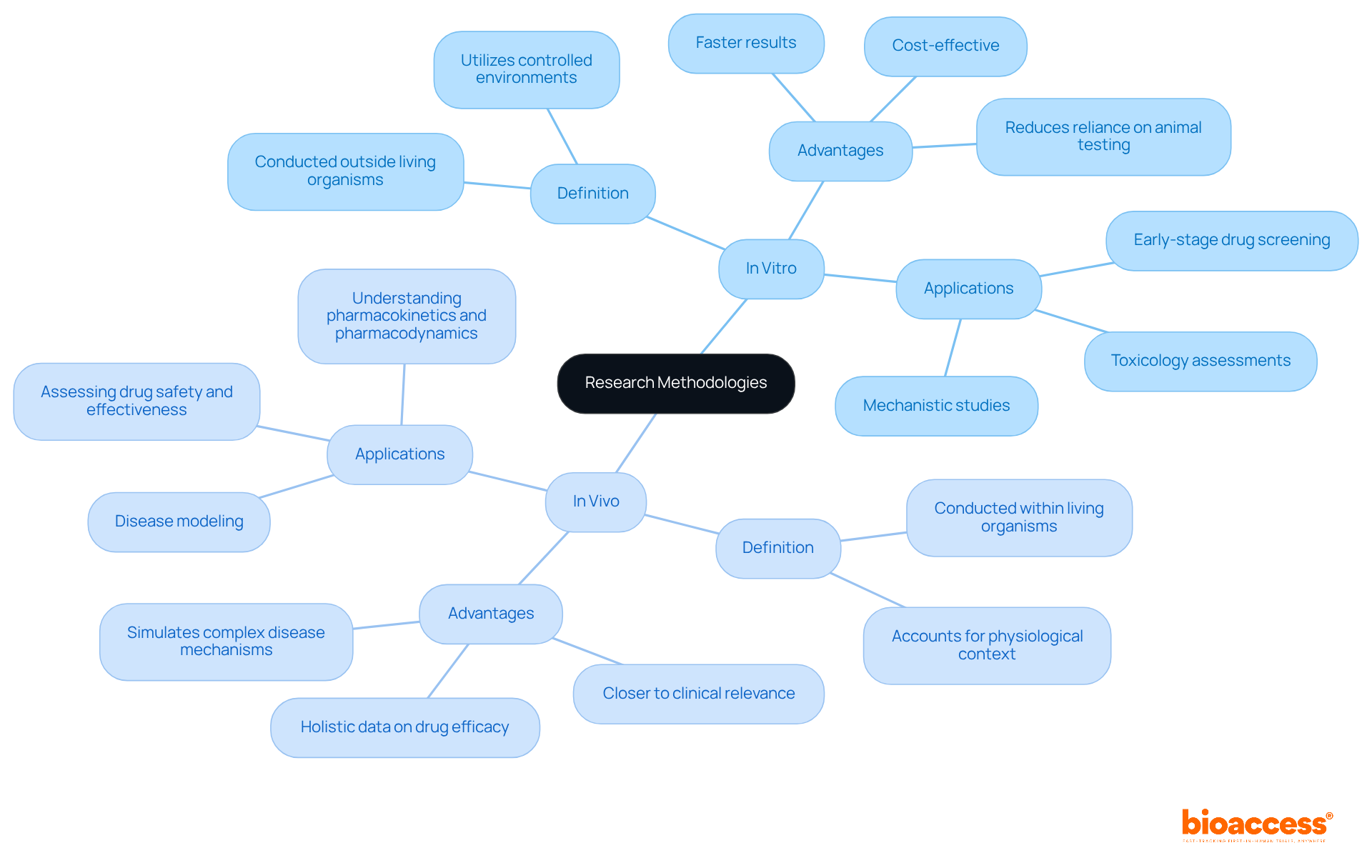
In vitro methodologies present several significant advantages that are crucial for clinical research:
However, it’s essential to recognize the limitations of in vitro studies:
On the other hand, in vivo methodologies offer distinct advantages:
Nevertheless, in vivo studies come with their own set of challenges:

In vitro methodologies are particularly suited for several key areas in drug development:
Conversely, in vivo methodologies play a crucial role in:
Ultimately, the decision between methodologies such as in vitro and in vivo depends on the specific inquiry, available resources, and ethical considerations. A balanced approach that combines both methodologies can produce the most thorough insights, enhancing the overall effectiveness of clinical studies.

Both in vitro and in vivo research methodologies, as well as laboratory practices, are governed by rigorous regulatory and ethical guidelines designed to safeguard human and animal welfare. Laboratory experiments typically face fewer regulatory hurdles since they do not involve living subjects; however, compliance with lab practices, data reporting standards, and safety protocols remains crucial. Bioaccess offers extensive clinical trial management services, including feasibility assessments and compliance evaluations, ensuring that assessments for in vitro and in vivo examinations meet necessary standards.
In contrast, research that is conducted in vitro and in vivo is subject to more intricate regulations due to the involvement of living organisms. Researchers must secure approval from Institutional Animal Care and Use Committees (IACUCs) or similar entities, which assess the justification, humane treatment, and scientific validity of proposed studies. Ethical considerations in this realm include minimizing animal suffering, employing the smallest number of animals necessary, and ensuring that all procedures are conducted by trained personnel. The 3Rs principles - Replacement, Reduction, and Refinement - are crucial for ethical and responsible animal studies.
Both methodologies must align with overarching regulatory frameworks such as Good Laboratory Practice (GLP) and Good Clinical Practice (GCP), which are essential for ensuring the reliability and integrity of study data in vitro and in vivo. As the biomedical exploration landscape evolves, ongoing dialogues regarding the ethical implications of these methodologies continue to influence regulatory practices, underscoring the necessity for responsible and transparent inquiry. Recent discussions have highlighted the importance of ethical guidelines for in vivo research, emphasizing the principles of Replacement, Reduction, and Refinement to enhance animal welfare while advancing scientific knowledge.
Furthermore, case studies such as "The Role of Ethics Committees in Research" illustrate the practical implications of these ethical considerations, showcasing how Ethics Committees ensure the protection of human rights and the well-being of research subjects. Katherine Ruiz, an expert in regulatory affairs for medical devices and in vitro diagnostics in Colombia, emphasizes the importance of compliance with INVIMA regulations to uphold these ethical standards.

In conclusion, the exploration of in vitro and in vivo research methodologies underscores the distinct yet complementary roles these approaches play in biomedical research. In vitro studies provide a controlled environment that facilitates early-stage drug discovery and mechanistic investigations, while in vivo methodologies offer insights into complex biological interactions observable only within living organisms. Recognizing these differences is crucial for researchers aiming to make informed decisions that align with their objectives and uphold ethical standards.
The key arguments presented throughout this article highlight the advantages and disadvantages of each methodology. In vitro research is lauded for its cost-effectiveness and ethical considerations, yet it struggles to replicate the complexities of living systems. On the other hand, in vivo research is invaluable for its physiological relevance and comprehensive data, although it demands greater resources and raises ethical concerns regarding animal welfare. A balanced approach that integrates both methodologies can yield the most thorough insights and enhance the effectiveness of clinical studies.
Ultimately, the ongoing dialogue surrounding regulatory and ethical considerations in research methodologies emphasizes the necessity for responsible practices that prioritize both scientific advancement and the welfare of research subjects. As the landscape of biomedical research continues to evolve, it is imperative for researchers to remain vigilant in adhering to ethical guidelines and regulatory standards, ensuring that their work not only contributes to scientific knowledge but also respects the intrinsic value of life, whether human or animal.
What is in vitro research methodology?
In vitro research methodology involves laboratory investigations conducted outside living organisms, typically in controlled environments like petri dishes or test tubes. It allows researchers to isolate specific biological components for detailed analysis.
What are the advantages of in vitro experiments?
In vitro experiments offer faster results, reduce reliance on animal testing, and are particularly beneficial for early-stage drug screening and mechanistic investigations.
What does in vivo research methodology entail?
In vivo research methodology involves experiments conducted within living organisms, providing a comprehensive understanding of biological processes in their natural context.
Why is in vivo research important?
In vivo research is crucial for assessing drug effectiveness, safety, and pharmacokinetics, as it accounts for the complex interactions within tissues and organs influenced by the physiological environment.
What are current trends in in vivo research methodologies?
Current trends include the integration of advanced technologies, such as organ-on-chip systems, to enhance the precision and ethical standards of in vivo studies.
How do in vitro and in vivo methodologies complement each other in biomedical research?
Both methodologies are indispensable in biomedical research, with in vitro experiments providing targeted insights into cellular mechanisms, while in vivo experiments are essential for understanding drug effects and biological responses in a living context.
What is the significance of adhering to regulations in biomedical research?
Adhering to regulations for medical devices and diagnostic tests ensures that innovations meet safety and efficacy standards, as emphasized by regulatory affairs specialists like Katherine Ruiz.