


In the realm of clinical research, Good Clinical Practice (GCP) stands as the cornerstone for ethical and scientific integrity, safeguarding the safety and rights of human participants. The recent surge in GCP audit triggers in Montenegro - evidenced by over 720 deficiencies identified during inspections - underscores an urgent need for Clinical Research Directors to bolster compliance measures. What strategies can be employed to navigate these challenges and transform audit findings into opportunities for improvement?
This article delves into the critical GCP audit triggers specific to Montenegro, offering insights and best practices to enhance research integrity and participant welfare. By addressing these pressing issues, we can not only ensure compliance but also foster a culture of continuous improvement in clinical research.
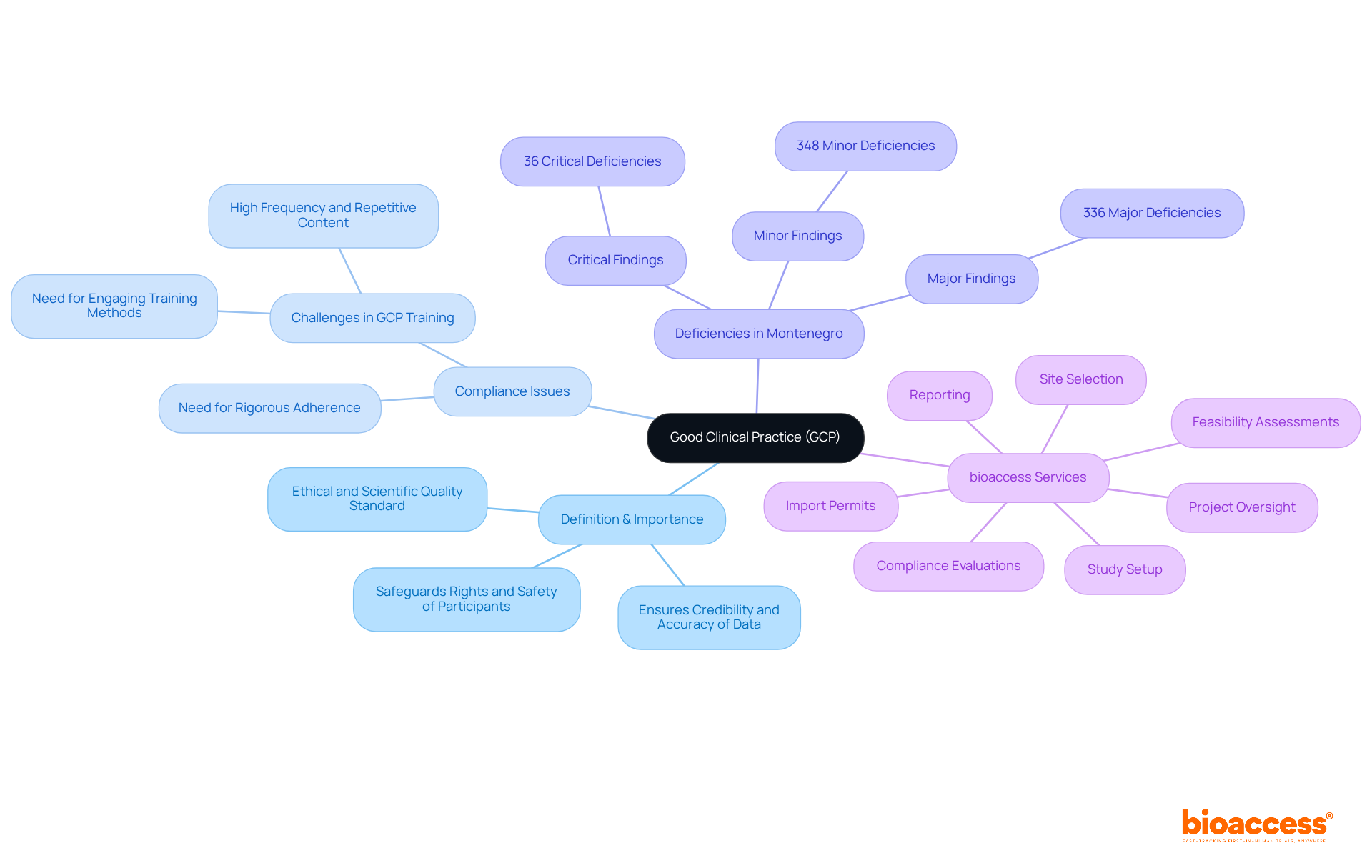
Good Clinical Practice (GCP) is an international ethical and scientific quality standard that is crucial for the design, conduct, recording, and reporting of studies involving human subjects. It safeguards the rights, safety, and well-being of participants while ensuring the credibility and accuracy of the data collected. Compliance with GCP is essential not only for maintaining public trust in clinical research but also as a prerequisite for regulatory approval, particularly concerning GCP audit triggers in Montenegro.
Recent inspections in Montenegro revealed a staggering total of 720 deficiencies, which are crucial GCP audit triggers in Montenegro, comprising 36 critical, 336 major, and 348 minor findings. This underscores the urgent need for rigorous adherence to GCP standards. By following GCP guidelines, Clinical Research Directors can conduct studies ethically, yielding reliable results that significantly contribute to advancements in medical knowledge and patient care.
bioaccess provides extensive clinical study management services, including:
These services are vital for ensuring GCP compliance. Furthermore, expert insights emphasize the necessity for ongoing improvements in GCP training, advocating for a shift from traditional check-box approaches to more engaging and practical training methods.
This evolution in GCP standards is essential for improving the quality of clinical studies and ensuring participant safety. Ultimately, it contributes to job creation, economic growth, and healthcare enhancement in the areas where these investigations are conducted.
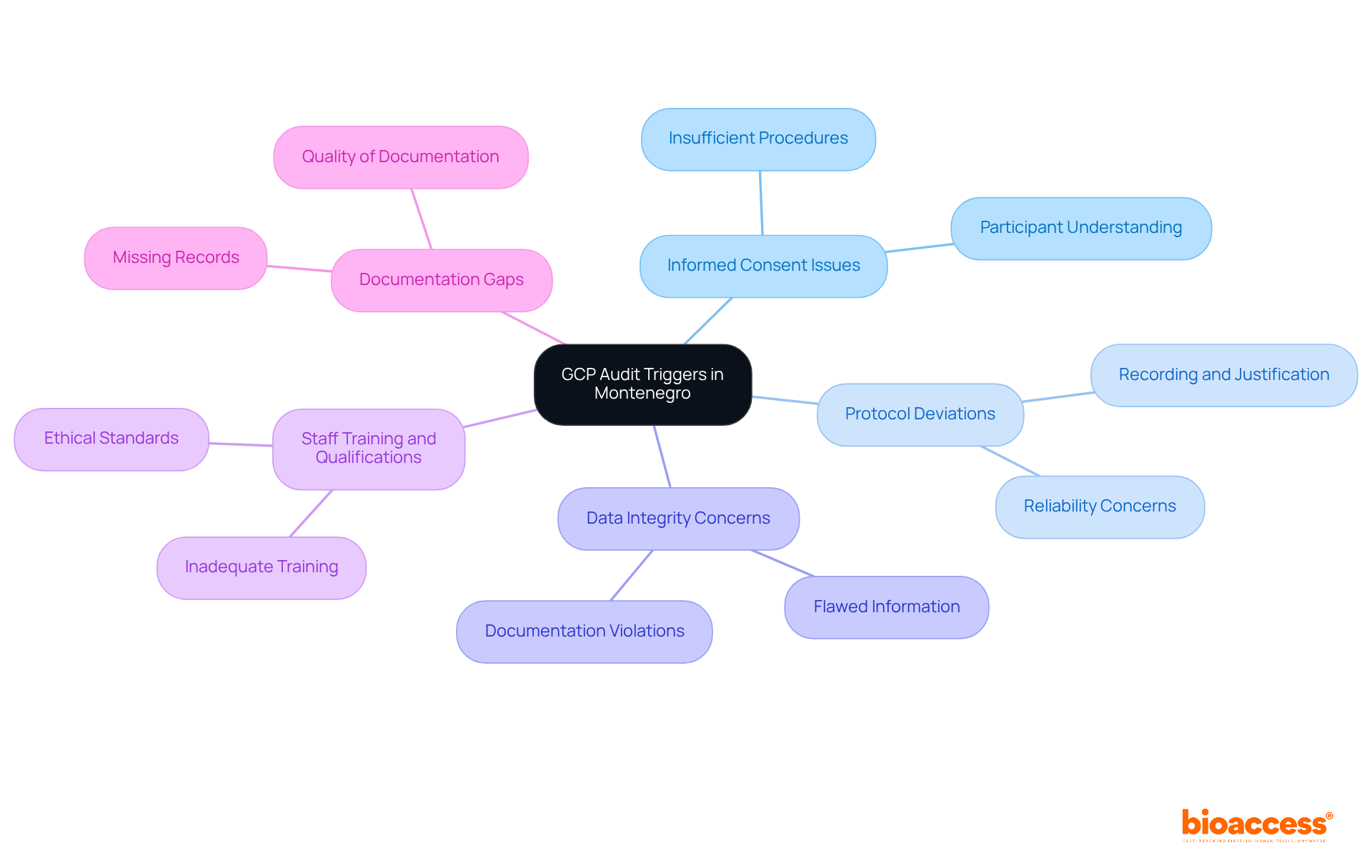
In Montenegro, the scrutiny during inspections is warranted due to several key GCP audit triggers in Montenegro. Understanding these triggers is essential for Clinical Research Directors aiming to uphold the integrity of clinical studies and protect participant welfare.
Informed Consent Issues: Insufficient informed consent procedures often represent a significant breach in clinical studies. Participants must fully comprehend the nature of the study, potential risks, and benefits before enrollment. Failing to provide comprehensive information can jeopardize participant safety and the overall integrity of the trial.
Protocol Deviations: Any departures from the approved study protocol must be meticulously recorded and justified. Frequent deviations can initiate reviews and raise concerns about the study's reliability, making it crucial to adhere strictly to established protocols.
Data Integrity Concerns: Flawed or incomplete information can trigger warnings during evaluations, underscoring the necessity for careful data management practices. Violations such as missing or falsified documentation can severely impact the credibility of trial data, leading to potential repercussions.
Staff Training and Qualifications: Inadequate training of research staff can lead to non-compliance. Ensuring that all team members are properly trained is vital for maintaining ethical standards and fostering a culture of accountability.
Documentation Gaps: Missing or inadequately maintained documentation can prompt reviews, emphasizing the significance of meticulous record-keeping. Proper documentation not only supports compliance but also enhances the overall quality of clinical research.
By recognizing the GCP audit triggers in Montenegro, Clinical Research Directors can take proactive measures to mitigate risks and enhance adherence. This ultimately safeguards participant welfare and reinforces the integrity of clinical research.
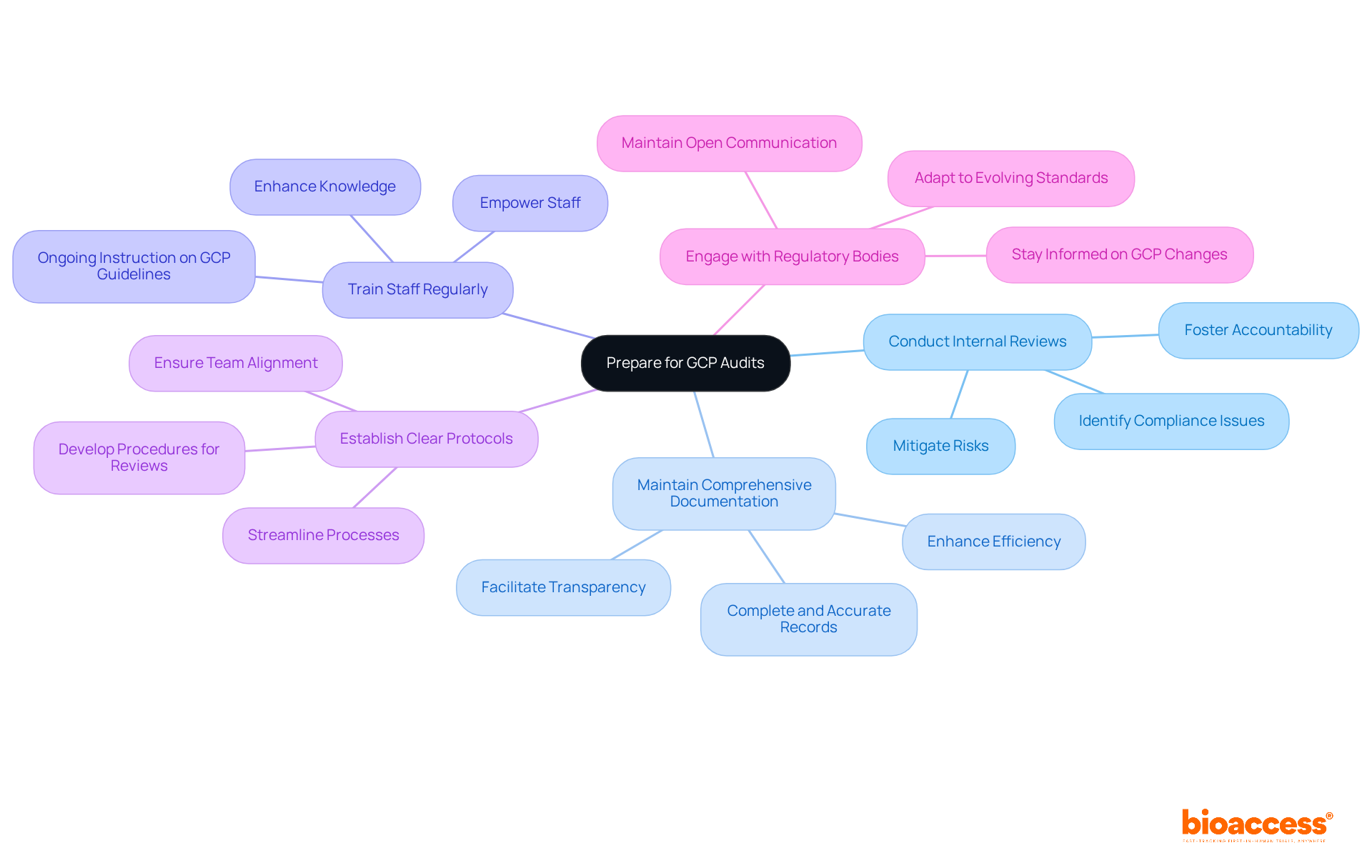
To prepare effectively for GCP evaluations, Clinical Research Directors must adopt key best practices that ensure compliance and readiness.
Conduct Internal Reviews: Regular internal reviews are essential for identifying potential compliance issues before external examinations occur. This proactive approach not only mitigates risks but also fosters a culture of accountability within the team.
Maintain Comprehensive Documentation: It is crucial to ensure that all study-related documents are complete, accurate, and easily accessible. Comprehensive documentation serves as the backbone of any successful clinical trial, facilitating transparency and efficiency.
Train Staff Regularly: Ongoing instruction on GCP guidelines and review processes is vital for all team members. Regular training sessions not only enhance knowledge but also empower staff to uphold the highest standards of compliance.
Establish Clear Protocols: Developing and communicating clear procedures for managing review requests and findings is imperative. This clarity helps streamline processes and ensures that everyone is on the same page during evaluations.
Engage with Regulatory Bodies: Maintaining open communication with regulatory authorities keeps Clinical Research Directors informed about any changes in GCP requirements. This engagement is crucial for adapting to evolving standards and ensuring ongoing compliance.
By adopting these strategies, Clinical Research Directors can significantly improve their readiness for evaluations and promote a culture of adherence within their teams.
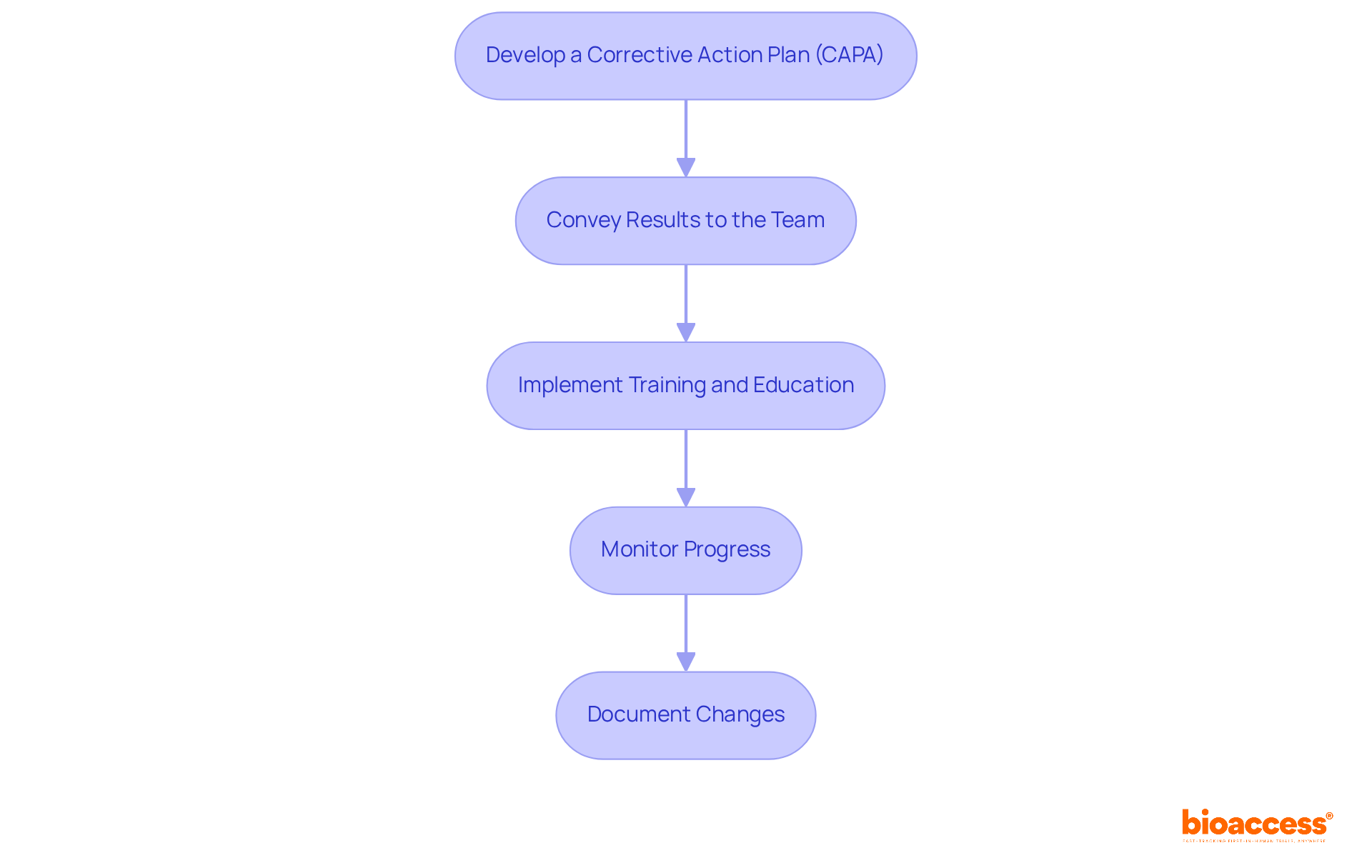
In response to audit findings, Clinical Research Directors must implement the following continuous improvement strategies:
By focusing on these strategies, Clinical Research Directors can transform audit findings into valuable opportunities for improving their clinical research operations, ultimately leading to better outcomes and adherence to regulatory standards.
Good Clinical Practice (GCP) is the bedrock of ethical and scientifically sound clinical research, safeguarding the safety and rights of participants while ensuring data integrity. In Montenegro, the importance of GCP compliance is highlighted by the concerning number of deficiencies identified in recent audits. By recognizing and addressing key GCP audit triggers, Clinical Research Directors can elevate the quality of their studies and protect participant welfare, thereby strengthening public trust in clinical research.
This article explores critical GCP audit triggers in Montenegro, such as:
Each of these factors presents significant risks to the credibility of clinical trials, underscoring the urgent need for strict adherence to GCP standards. Furthermore, implementing best practices-like regular internal reviews, thorough documentation, ongoing staff training, and proactive engagement with regulatory bodies-can greatly enhance compliance and preparedness for audits.
Given these insights, it is essential for Clinical Research Directors to perceive GCP compliance not just as a regulatory obligation but as a chance for continuous improvement. By adopting a proactive approach and cultivating a culture of accountability, the clinical research community can improve study quality, contribute to medical advancements, and ultimately enhance patient care. Embracing these principles will not only ensure compliance but also lay the groundwork for a more robust and ethical framework in clinical research practices.
What does GCP stand for and what is its significance in clinical research?
GCP stands for Good Clinical Practice, which is an international ethical and scientific quality standard essential for the design, conduct, recording, and reporting of studies involving human subjects. It is significant because it safeguards the rights, safety, and well-being of participants while ensuring the credibility and accuracy of the data collected.
Why is compliance with GCP important?
Compliance with GCP is crucial for maintaining public trust in clinical research and is a prerequisite for regulatory approval. It also helps in identifying and addressing deficiencies in clinical studies, as highlighted by recent inspections revealing numerous deficiencies in Montenegro.
What were the findings of the recent GCP inspections in Montenegro?
Recent inspections in Montenegro uncovered a total of 720 deficiencies, which included 36 critical, 336 major, and 348 minor findings. This highlights the urgent need for adherence to GCP standards.
What services does bioaccess provide to support GCP compliance?
Bioaccess offers extensive clinical study management services, including feasibility assessments, site selection, compliance evaluations, study setup, import permits, project oversight, and reporting, all of which are vital for ensuring GCP compliance.
What improvements are suggested for GCP training?
Experts emphasize the need for ongoing improvements in GCP training, advocating for a shift from traditional check-box approaches to more engaging and practical training methods.
How does adherence to GCP standards impact clinical studies and the broader community?
Adhering to GCP standards improves the quality of clinical studies and ensures participant safety. This, in turn, contributes to job creation, economic growth, and healthcare enhancement in the areas where these investigations are conducted.