


The article offers critical insights into the significance of the Fab region in clinical research, underscoring its essential role in antibody specificity, therapeutic efficacy, and advancements in protein engineering. It elucidates how modifications to the Fab region can enhance binding capabilities and improve treatment outcomes, illustrated by successful therapies such as infliximab. Furthermore, it emphasizes the importance of ongoing research in immune protein engineering, which is vital for addressing future challenges in the field.
The Fab region, a fundamental component of antibodies, is pivotal in determining the specificity and efficacy of immune responses. This makes it a focal point in clinical research and therapeutic development. By delving into its intricate structure and functional significance, researchers can unlock the potential for enhanced treatments across various diseases.
However, as advancements in protein engineering continue to evolve, the challenge remains: how can the Fab region be optimized to maximize therapeutic outcomes while minimizing side effects? Exploring this tension not only sheds light on current trends but also paves the way for future innovations in antibody therapeutics.
The Fab (fragment antigen-binding) portion serves as a critical component of immunoglobulins, responsible for the identification and attachment to specific antigens. Structurally, it comprises two identical arms, each containing a light chain alongside a segment of a heavy chain. Each heavy chain consists of four domains—one variable and three constant—while each light chain contains two domains—one variable and one constant. This precise configuration facilitates the specificity of antigen recognition, which is vital for the efficacy of therapeutic agents, positioning the Fab region as a central focus in drug development and clinical research.
Recent studies indicate that designing proteins with enhanced Fab segments can significantly improve binding capabilities, thereby elevating treatment outcomes across various diseases. For example, infliximab has proven to be an effective treatment for inflammatory bowel disease and rheumatoid arthritis, highlighting the crucial role of the Fab region in targeting specific antigens.
Additionally, understanding the function of autoantibodies in autoimmune disorders provides deeper insights into the importance of specificity. This understanding not only aids in the development of more effective immune proteins but also supports ongoing research aimed at enhancing therapeutic applications within the medical field.
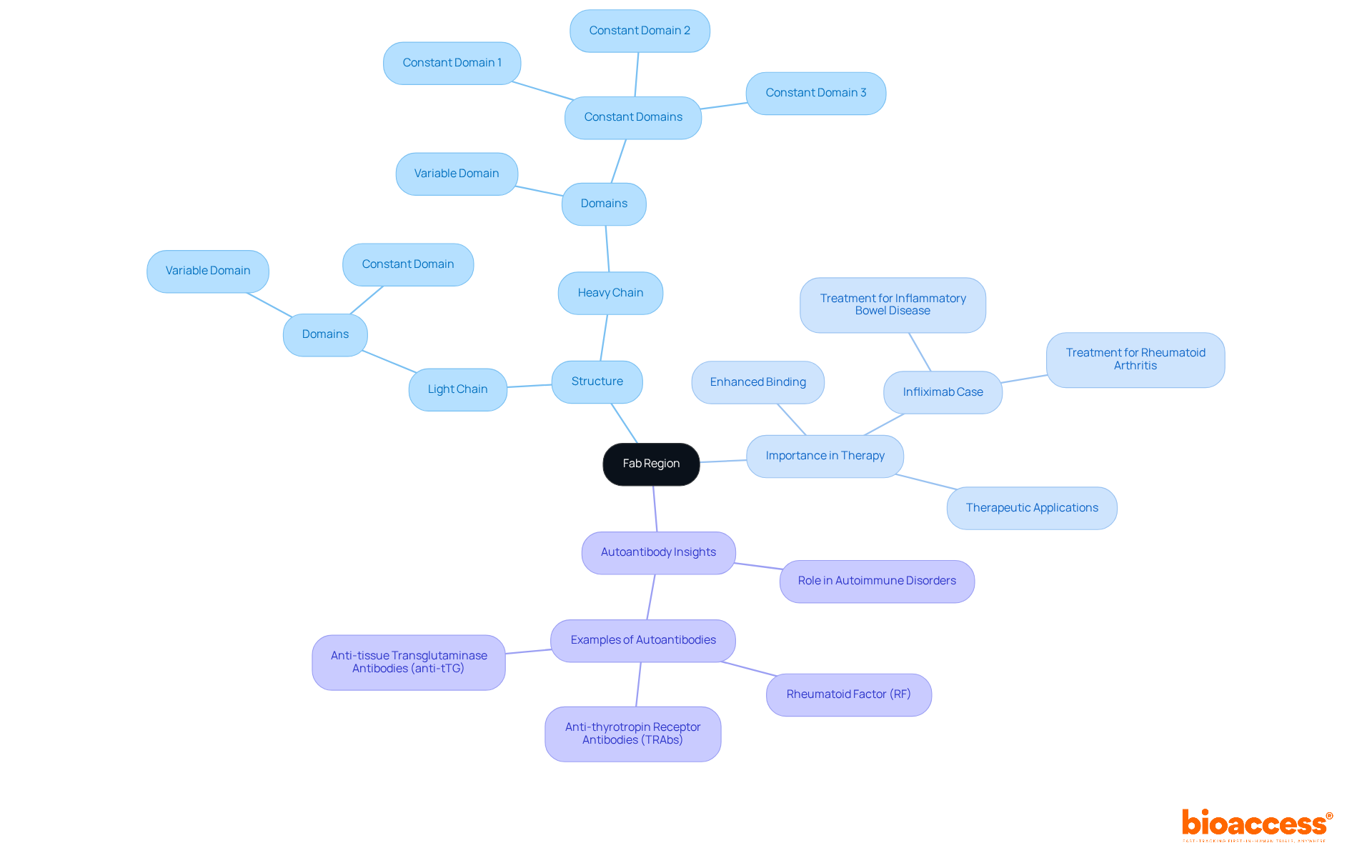
The fab region consists of two primary structural components: the variable section (V) and the constant section (C). The variable area, which includes the light chain (VL) and the heavy chain (VH), is crucial for the unique specificity of the immune protein, allowing it to bind to a specific antigen. In contrast, the constant region (CL and CH1) contributes to structural stability and the overall integrity of the immune protein. The arrangement of these domains creates a distinct three-dimensional structure essential for the molecule's effective recognition and attachment to its target antigen. Understanding these elements is vital for researchers in the fab region who are seeking to modify or enhance immune protein functions for medical applications.
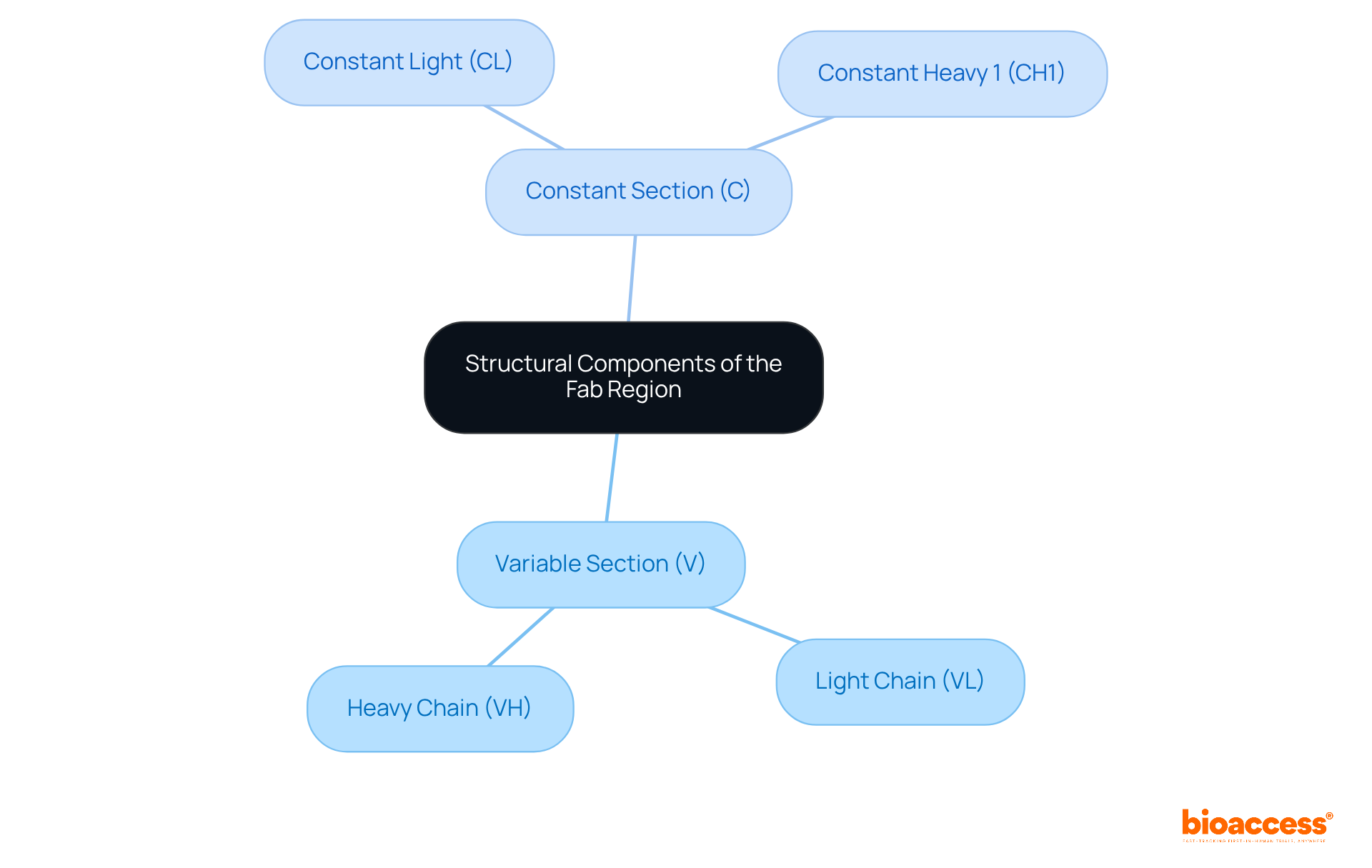
The fab region is pivotal to the efficacy of antibodies, as it facilitates attachment to specific antigens essential for triggering immune responses. This binding can lead to diverse therapeutic outcomes, such as:
For example, in cancer therapies, proteins with optimized binding sites have demonstrated enhanced selectivity for tumor cells, significantly reducing collateral damage to healthy tissues. However, higher affinity immunoglobulins may face limited penetration into tumor mass due to the binding site barrier effect, posing a challenge for effective treatment.
Statistics reveal that high-affinity proteins can achieve up to 90% binding efficacy in targeted therapies, underscoring the importance of specificity in the fab region for treatment effectiveness. Beginning in 2025, advancements in immune protein engineering will focus on enhancing the binding properties of the fab region, which is expected to improve overall treatment outcomes. Furthermore, the medicinal monoclonal protein market is projected to generate substantial revenue, reflecting the growing significance of these therapies. Understanding these functional roles is crucial for researchers dedicated to advancing next-generation immune therapies that enhance efficacy while minimizing side effects.
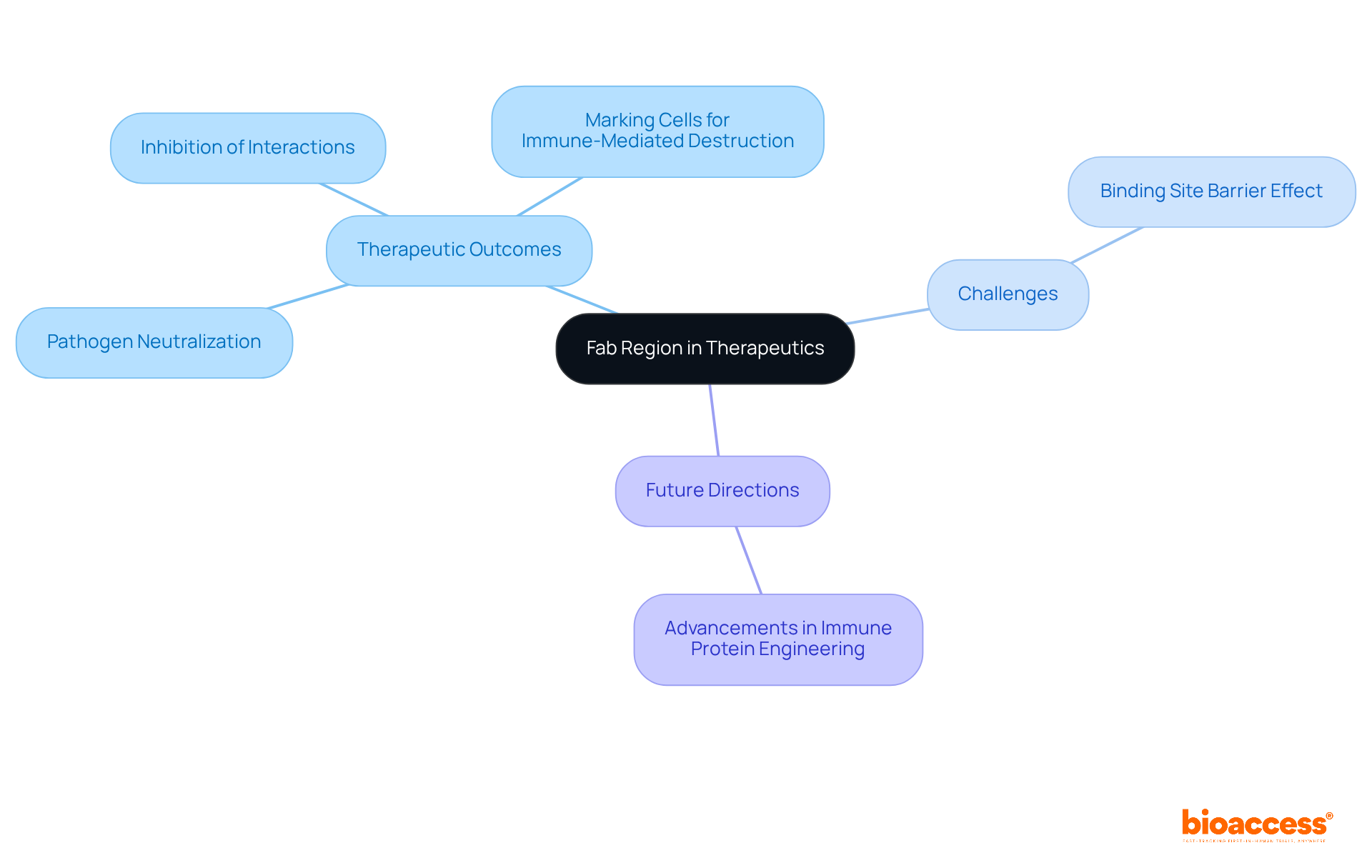
Engineering and modifications of the fab region are crucial for enhancing the therapeutic effectiveness of antibodies. Affinity maturation, a fundamental technique, involves repeated cycles of mutation and selection aimed at improving the binding affinity of the fab region. This process frequently encompasses the refinement of complementarity-determining regions (CDRs) to increase specificity, alongside the introduction of mutations that bolster stability while minimizing immunogenicity.
As Alyssa Shepard notes, optimizing immune proteins for improved pharmacokinetics and reduced immunogenicity facilitates more efficient and targeted delivery of therapies, ultimately amplifying treatment effectiveness. These engineering strategies not only enable the production of proteins that effectively target specific antigens but also enhance their pharmacokinetic properties, leading to improved patient outcomes.
Recent advancements in immune protein modifications have demonstrated notable success rates in developing treatments that are both more effective and safer for patients. For instance, the affinity maturation process can yield proteins exhibiting tens to thousands-fold improvements in antigen-binding capacity within just one or two rounds of maturation cycles.
As the landscape of antibody therapeutics evolves, a comprehensive understanding of these engineering techniques will be essential for researchers dedicated to innovating and optimizing antibody-based treatments.
The Fab region plays a pivotal role in the functionality of antibodies, serving as the primary binding site for specific antigens. Its intricate structure, consisting of variable and constant domains, is essential for therapeutic effectiveness, influencing the design and development of advanced immunotherapies. By understanding the nuances of the Fab region, clinical research leaders are better equipped to innovate and enhance treatment outcomes in various medical applications.
Key insights reveal that the engineering and modification of the Fab region are crucial for improving binding affinity and specificity, which directly impacts therapeutic efficacy. Techniques such as affinity maturation and the optimization of complementarity-determining regions are instrumental in creating antibodies that not only target specific antigens more effectively but also minimize side effects. As the field progresses, anticipated advancements in immune protein engineering are expected to further elevate treatment effectiveness and patient safety.
Ultimately, the significance of the Fab region in clinical research cannot be overstated. As researchers continue to explore and refine its properties, there lies an opportunity to revolutionize therapeutic approaches across numerous diseases. Embracing these insights and engineering innovations will be vital for driving the future of antibody therapeutics, making a meaningful impact on patient care and treatment efficacy.
What is the Fab region and what is its role?
The Fab (fragment antigen-binding) region is a critical component of immunoglobulins responsible for identifying and attaching to specific antigens.
What is the structure of the Fab region?
The Fab region comprises two identical arms, each containing a light chain and a segment of a heavy chain. Each heavy chain has four domains (one variable and three constant), while each light chain contains two domains (one variable and one constant).
Why is the specificity of the Fab region important?
The specificity of the Fab region is vital for effective antigen recognition, which is essential for the efficacy of therapeutic agents and plays a central role in drug development and clinical research.
How can enhancing the Fab region improve treatment outcomes?
Designing proteins with enhanced Fab segments can significantly improve binding capabilities, leading to better treatment outcomes across various diseases.
Can you provide an example of a therapeutic agent that utilizes the Fab region?
Infliximab is an example of a therapeutic agent that effectively targets specific antigens, proving beneficial in treating inflammatory bowel disease and rheumatoid arthritis.
How does understanding autoantibodies relate to the Fab region?
Understanding the function of autoantibodies in autoimmune disorders provides insights into the importance of specificity, aiding in the development of more effective immune proteins and enhancing therapeutic applications in medicine.