

The article delineates four essential practices for mastering Clinical Trial Authorization (CTA) regulatory compliance:
These practices are substantiated by evidence indicating that effective navigation of the CTA process can significantly enhance the likelihood of successful study initiation and adherence to regulatory standards. Ultimately, this leads to more efficient research outcomes, underscoring the critical nature of these practices in the realm of clinical research.
Navigating the complexities of Clinical Trial Authorization (CTA) is essential for organizations aiming to conduct research involving human participants. Mastering the regulatory landscape not only ensures compliance but also accelerates the path to market for innovative treatments. However, with evolving standards and stringent oversight, how can stakeholders effectively align their practices to meet these challenges? This article explores four essential practices that can empower organizations to conquer CTA regulatory compliance, ultimately enhancing the success of clinical trials and safeguarding participant welfare.
The CTA regulatory procedure is essential for initiating research studies, as it requires consent from oversight bodies to conduct investigations involving human participants. To navigate this process effectively, stakeholders should consider several key practices:
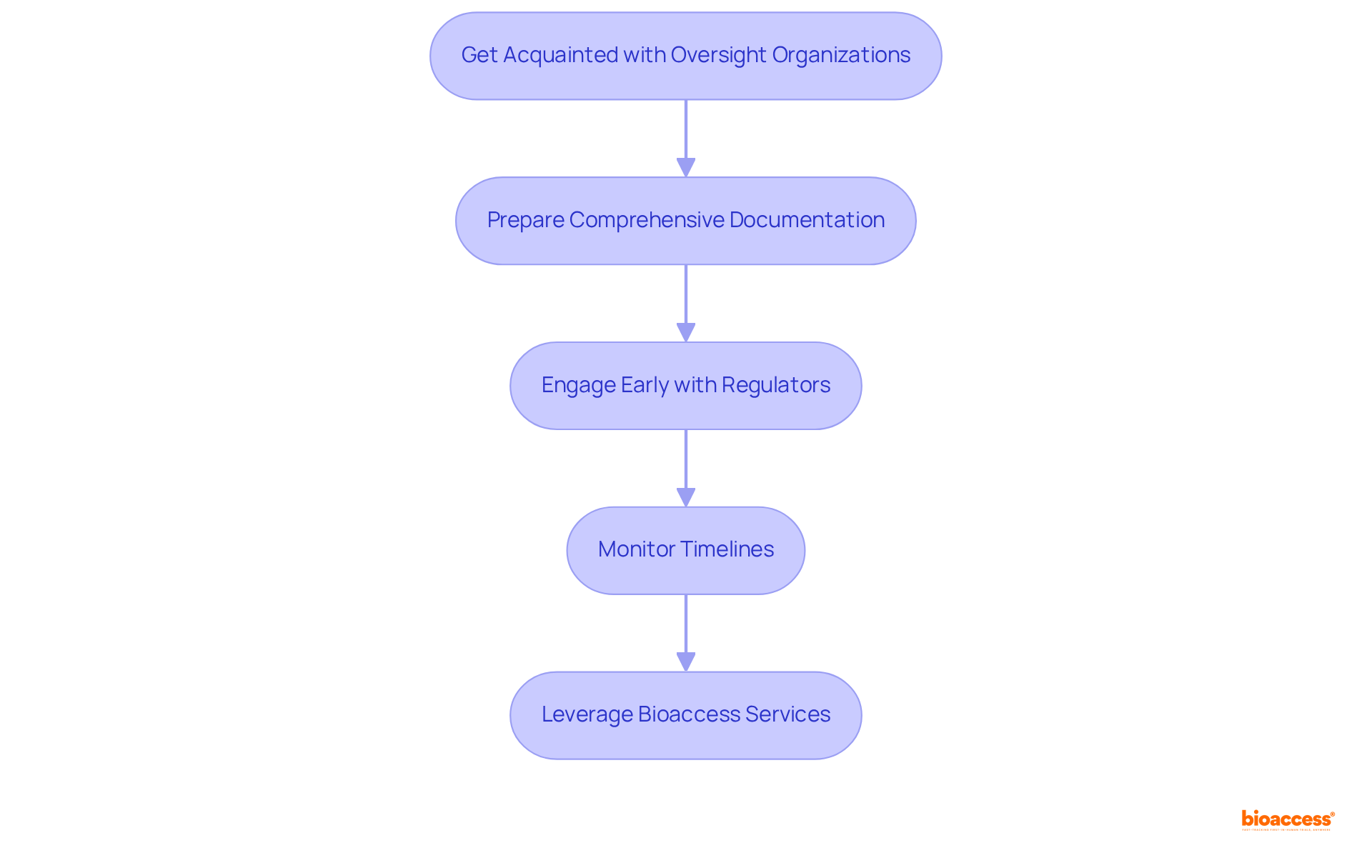
Get Acquainted with Oversight Organizations: Identify the oversight authorities governing clinical trials in your region, such as the FDA in the U.S. or the EMA in Europe, to grasp their specific requirements and guidelines. Bioaccess® can assist by providing insights into local regulations and facilitating communication with these bodies.
Prepare Comprehensive Documentation: Compile all necessary documents meticulously, including the study protocol, informed consent forms, and investigator brochures, ensuring compliance with standards to facilitate a smooth review process. Bioaccess® offers support in document preparation and compliance reviews to ensure all materials are properly organized.
Engage Early with Regulators: Initiate discussions with regulatory bodies during the planning phase to clarify expectations and requirements. An acknowledgment letter is issued to sponsors following application screening, marking the start of the review period, which is a critical step in the process. Bioaccess® can facilitate these early engagements to streamline the CTA regulatory approval process.
Monitor Timelines: Vigilantly track submission and approval timelines, as delays can significantly impact the study's commencement. Recent data indicates that research study applications have been evaluated within 30 days since September 2023, with 98% of studies receiving a combined review decision within 60 days. This underscores the importance of prompt submissions. Furthermore, the typical duration for evaluations related to research studies is 28 days for initial assessments, providing stakeholders with clearer expectations. Bioaccess®'s project management services ensure timelines are monitored and adhered to, enhancing the efficiency of the CTA regulatory process.
By leveraging Bioaccess®'s extensive research study management services—including feasibility assessments, site selection, adherence reviews, study setup, import permits, project oversight, and reporting—organizations can master the CTA regulatory process. Katherine Ruiz, a specialist in oversight matters for medical devices and in vitro diagnostics in Colombia, offers invaluable insights to ensure adherence to standards. Additionally, employing Bioaccess®'s services can expedite research studies, reducing the time to market for new treatments and improving the effectiveness of research initiatives.
To ensure compliance with the CTA, it is essential to identify and adhere to key regulatory standards, including:
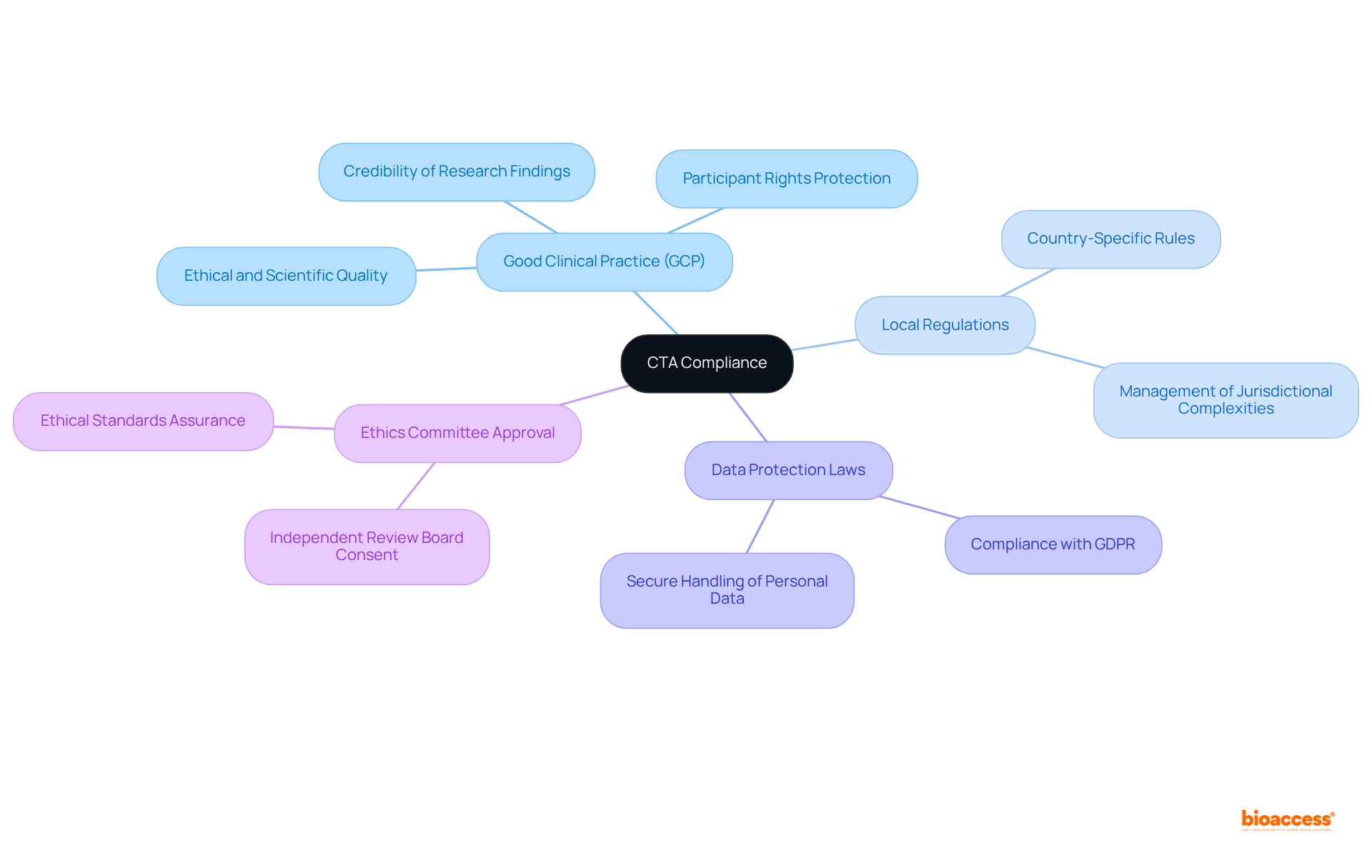
Good Clinical Practice (GCP): Comprehending GCP guidelines is crucial, as they set the ethical and scientific quality benchmarks for the design, execution, and reporting of research studies. Compliance with these guidelines not only protects participant rights but also enhances the credibility of research findings. As emphasized by industry experts, GCP serves as a vital framework that ensures studies are conducted ethically and that participant safety is prioritized.
Local Regulations: Each nation has specific rules governing clinical studies, which can differ greatly. Acquaintance with these local regulations is essential for adherence and can assist organizations in managing the intricacies of performing assessments in various jurisdictions. For example, in Latin America and Europe, regulatory frameworks may include unique conditions that must be fulfilled to enable successful execution of the study.
Data Protection Laws: Compliance with data protection regulations, such as the General Data Protection Regulation (GDPR) in Europe, is imperative. These laws govern the handling of personal data in clinical research, ensuring that participant information is managed securely and ethically. Organizations must implement robust data management practices to align with these legal requirements.
Ethics Committee Approval: Obtaining consent from an independent ethics committee or institutional review board (IRB) is a crucial step in the research process. This endorsement guarantees that the study meets ethical standards and that participant welfare is prioritized throughout the research.
By identifying and adhering to these CTA regulatory standards, organizations can mitigate risks associated with non-compliance, which can lead to significant financial penalties and reputational damage. Moreover, a systematic method for GCP compliance enhances public confidence and promotes involvement in research studies, ultimately aiding the progress of medical knowledge.
Recruitment is a crucial element of clinical studies, and enhancing these efforts can significantly influence study outcomes. To that end, consider the following key strategies:
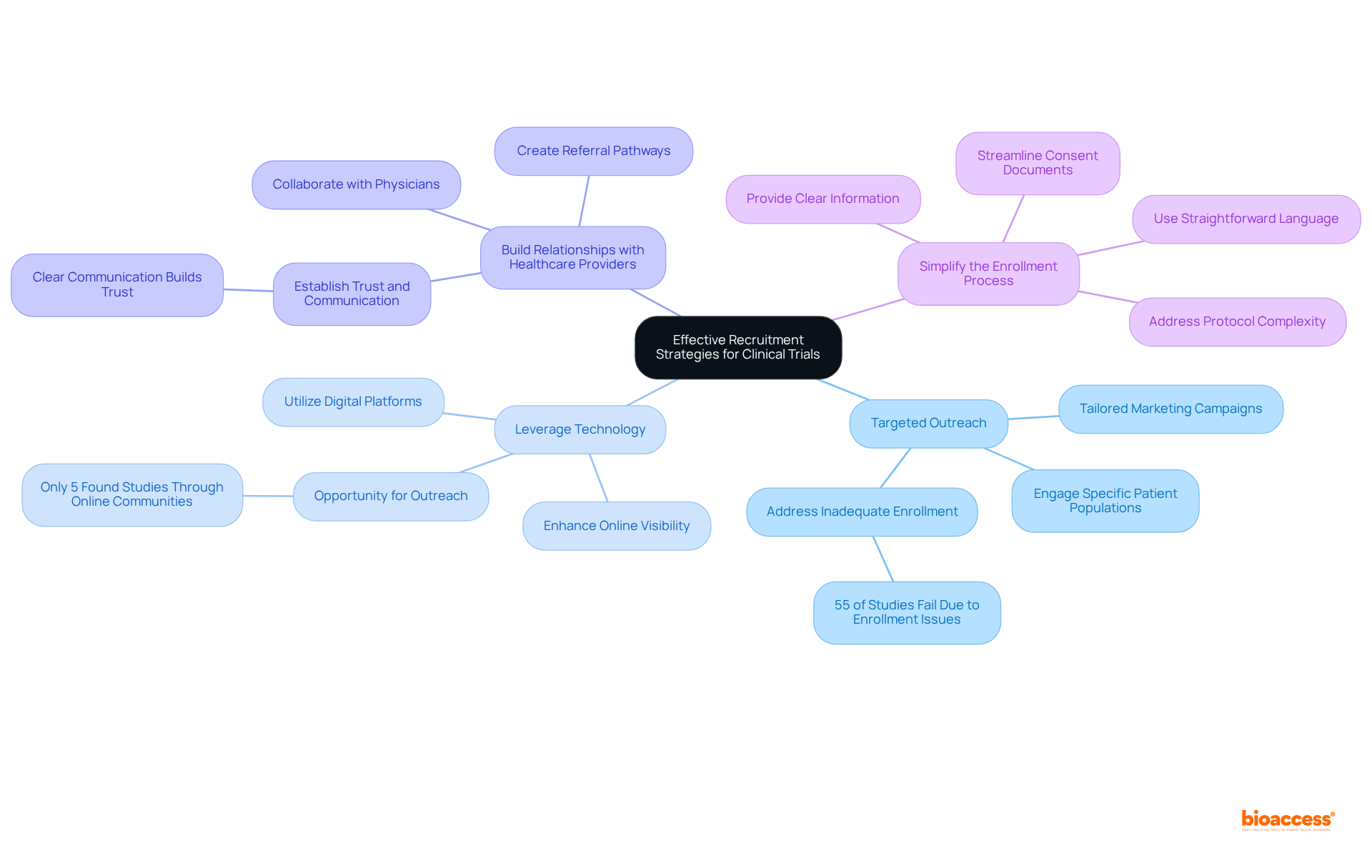
Targeted Outreach: Engage specific patient populations that align with study criteria through tailored marketing campaigns. This approach ensures that messaging resonates with potential participants, thereby increasing the likelihood of enrollment. Notably, 55% of studies globally do not succeed due to inadequate enrollment, underscoring the necessity of efficient outreach.
Leverage Technology: Utilize digital platforms and social media to broaden reach and facilitate access to information about the examination. By enhancing online visibility, prospective participants can easily discover and learn about available studies, thus improving recruitment efforts. For instance, only 5% of research participants found studies through online patient communities, indicating a significant opportunity for outreach.
Build Relationships with Healthcare Providers: Collaborate with physicians and healthcare organizations to create referral pathways for eligible patients. Establishing trust and communication with healthcare providers can lead to increased patient involvement in research studies. Experts note, "Clear communication builds trust, and when recruitment materials plainly state the benefits and risks, participants can make informed decisions."
Simplify the Enrollment Process: Streamline the enrollment process to minimize barriers for participants. This entails simplifying consent documents, utilizing straightforward language, and providing clear, accessible information regarding the study's purpose and procedures. Addressing common pitfalls, such as protocol complexity, is crucial for enhancing recruitment outcomes.
Implementing these strategies can accelerate enrollment rates and contribute to the overall success of medical studies, ensuring that innovative therapies reach the market more effectively. Furthermore, it is essential to emphasize the importance of diversity in clinical trial recruitment, as African Americans represent 12% of the U.S. population but only 5% of clinical trial participants.
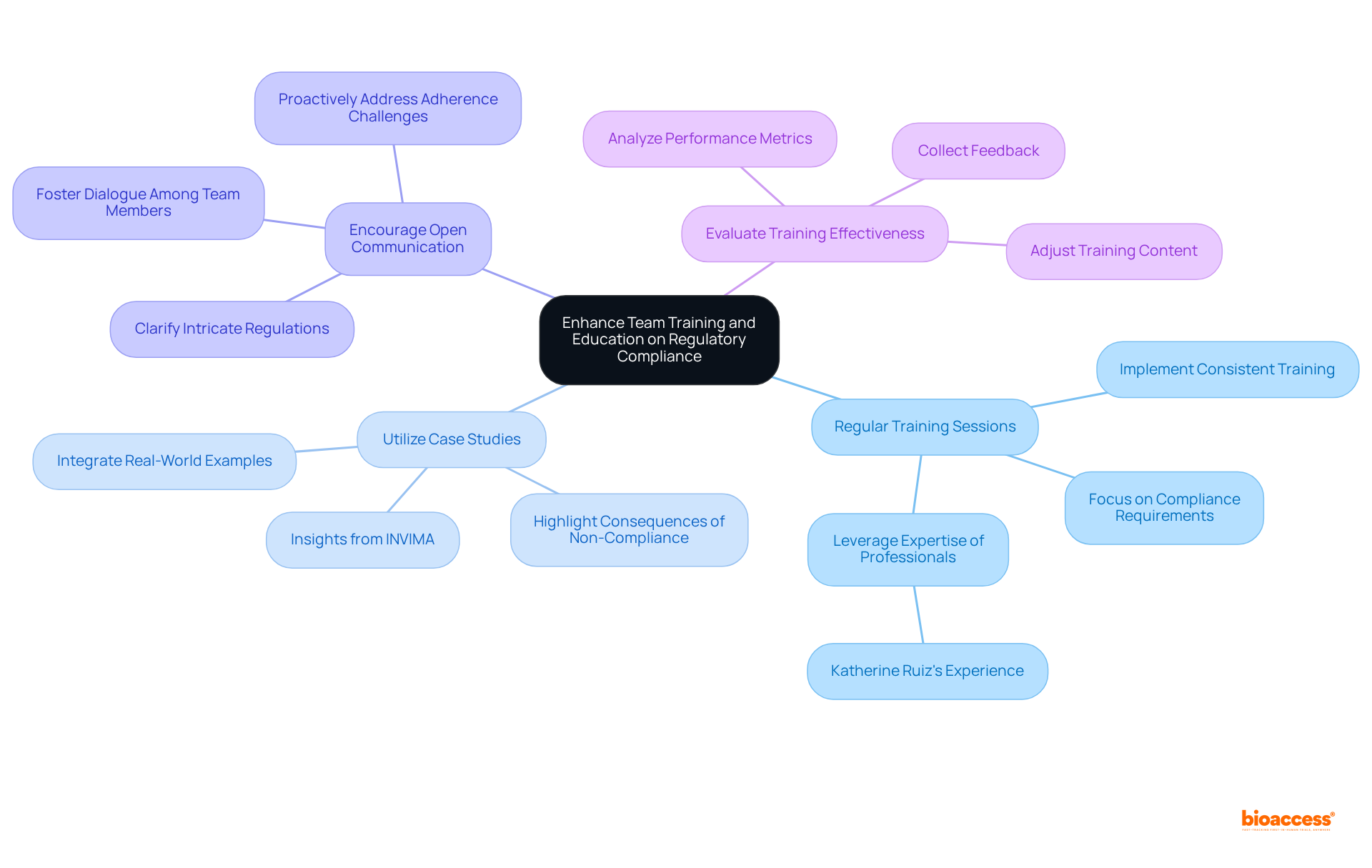
To enhance regulatory compliance, organizations must prioritize team training and education through several critical practices:
Regular Training Sessions: Organizations should implement consistent training sessions focused on compliance requirements, Good Clinical Practice (GCP), and ethical considerations. This approach ensures that the team remains informed about the latest cta regulatory standards while fostering a culture of ongoing learning. Leveraging the expertise of professionals like Katherine Ruiz, who has extensive experience in oversight for medical devices and in vitro diagnostics, can significantly enhance these training sessions, particularly regarding study setup and adherence evaluations provided by bioaccess.
Utilize Case Studies: Integrating real-world case studies into training programs is essential to illustrate the repercussions of non-compliance. Organizations that have faced cta regulatory failures often highlight the financial and reputational damage incurred, emphasizing the necessity of adhering to regulations. Katherine's experience with INVIMA, Colombia's national regulatory authority, provides valuable insights into the cta regulatory landscape that can be shared in these case studies, especially in the context of bioaccess's comprehensive clinical trial management services.
Encourage Open Communication: Creating an environment that fosters open dialogue among team members regarding regulatory matters is vital. Encouraging inquiries and discussions helps clarify intricate regulations and enables personnel to proactively address potential adherence challenges. This transparent communication is crucial for developing a knowledgeable team well-versed in the nuances of cta regulatory compliance, particularly in medical studies and the services offered by bioaccess.
Evaluate Training Effectiveness: Regular assessment of training initiatives through feedback collection and performance metrics analysis is necessary. Adjusting training content and methods based on these evaluations ensures alignment with evolving regulations and organizational needs. Continuous improvement of training programs is essential for maintaining standards, particularly as cta regulatory changes and new challenges arise in medical research.
Investing in comprehensive team training equips clinical research professionals with the essential knowledge and skills to uphold compliance. This commitment ultimately contributes to the success of clinical trials and enhances the service capabilities of bioaccess.
Mastering compliance with Clinical Trial Authorization (CTA) is essential for the successful initiation and execution of clinical research. Understanding the intricacies of the CTA process enables organizations to navigate regulatory requirements effectively, ensuring that studies proceed smoothly and ethically. The importance of adhering to these standards cannot be overstated; they safeguard participant welfare and enhance the credibility of research findings.
Key practices highlighted in the article include:
Additionally, understanding key regulatory standards such as:
is vital for maintaining compliance. Furthermore, the article emphasizes the significance of effective recruitment strategies and continuous team training to foster a culture of compliance and awareness within organizations.
Ultimately, adherence to CTA regulatory compliance not only mitigates risks associated with non-compliance but also promotes public confidence in clinical research. By leveraging the expertise of organizations like Bioaccess® and implementing the best practices outlined, stakeholders can streamline their processes, enhance participant recruitment, and expedite the journey of new treatments to market. Prioritizing these practices will benefit individual studies and contribute to the overall advancement of medical knowledge and patient care.
What is the Clinical Trial Authorization (CTA) process?
The CTA process is a regulatory procedure essential for initiating research studies involving human participants, requiring consent from oversight bodies.
Why is it important to understand oversight organizations in the CTA process?
Understanding oversight organizations, such as the FDA in the U.S. or the EMA in Europe, is crucial to grasp their specific requirements and guidelines for conducting clinical trials.
How can Bioaccess® assist with the CTA process?
Bioaccess® provides insights into local regulations, facilitates communication with oversight bodies, and offers support in document preparation and compliance reviews.
What documentation is necessary for the CTA process?
Necessary documentation includes the study protocol, informed consent forms, and investigator brochures, all of which must comply with regulatory standards.
When should stakeholders engage with regulators during the CTA process?
Stakeholders should engage with regulators during the planning phase to clarify expectations and requirements, which helps streamline the approval process.
What is the significance of monitoring timelines in the CTA process?
Monitoring submission and approval timelines is vital, as delays can significantly impact the study's commencement. Recent data shows that most research study applications are evaluated within 30 days.
What are the typical evaluation durations for research study applications?
The typical duration for initial assessments related to research studies is 28 days, with 98% of studies receiving a review decision within 60 days.
How can Bioaccess® enhance the efficiency of the CTA regulatory process?
Bioaccess® offers project management services to ensure timelines are monitored and adhered to, enhancing the overall efficiency of the CTA process.
Who is Katherine Ruiz and what role does she play in the CTA process?
Katherine Ruiz is a specialist in oversight matters for medical devices and in vitro diagnostics in Colombia, providing valuable insights to ensure adherence to regulatory standards.
What benefits can organizations gain by leveraging Bioaccess®'s services?
By using Bioaccess®'s services, organizations can expedite research studies, reduce time to market for new treatments, and improve the effectiveness of their research initiatives.