


The article underscores the medical significance of four pivotal radiopharmaceuticals, delineating their essential roles in diagnosis and treatment within the realm of nuclear medicine. It asserts that these specialized drugs, including [Lu]Lu-PSMA-617 and [177Lu]Lu-DOTA-TATE, are indispensable for targeted therapies and imaging modalities such as PET and SPECT. These techniques significantly enhance the precision of disease diagnosis and treatment, particularly for conditions such as cancer. Through the lens of nuclear medicine, the importance of these radiopharmaceuticals cannot be overstated.
The world of medicine has been revolutionized by the advent of radiopharmaceuticals—specialized drugs that harness the power of radioactive isotopes to transform diagnostic and therapeutic practices.
With over 60 approved agents, these innovative compounds enhance imaging techniques like PET and SPECT while targeting complex conditions such as cancer and heart disease, thus offering new hope to patients.
However, as the field rapidly evolves, questions arise regarding the safety, accessibility, and future of these critical medical tools.
What are the key examples of radiopharmaceuticals shaping modern healthcare, and how do they balance efficacy with safety in an ever-changing landscape?
An example of radiopharmaceuticals is a specialized class of drugs that incorporate radioactive isotopes, playing a pivotal role in both diagnostic and therapeutic applications within medicine. Their significance in nuclear medicine is underscored by their capacity to deliver targeted radiation to specific tissues, facilitating accurate imaging and management of various medical conditions, particularly tumors. Currently, there are 67 radioactive pharmaceuticals authorized globally, with 54 designated for diagnosis and 13 for treatment, addressing a spectrum of conditions including tumors, neurodegenerative disorders, and heart diseases. Techniques such as Positron Emission Tomography (PET) and Single Photon Emission Computed Tomography (SPECT) leverage these agents to produce detailed images of the body's internal structures, thereby enhancing disease diagnosis and management.
As we approach 2025, the integration of radioactive drugs into clinical practice continues to evolve, with emerging trends highlighting combination therapies and innovative agents aimed at improving treatment effectiveness and patient outcomes. Noteworthy radiopharmaceuticals examples include:
These examples illustrate the tangible impact of these innovative therapies in oncology, reinforcing the importance of ongoing research and collaboration in advancing clinical applications.
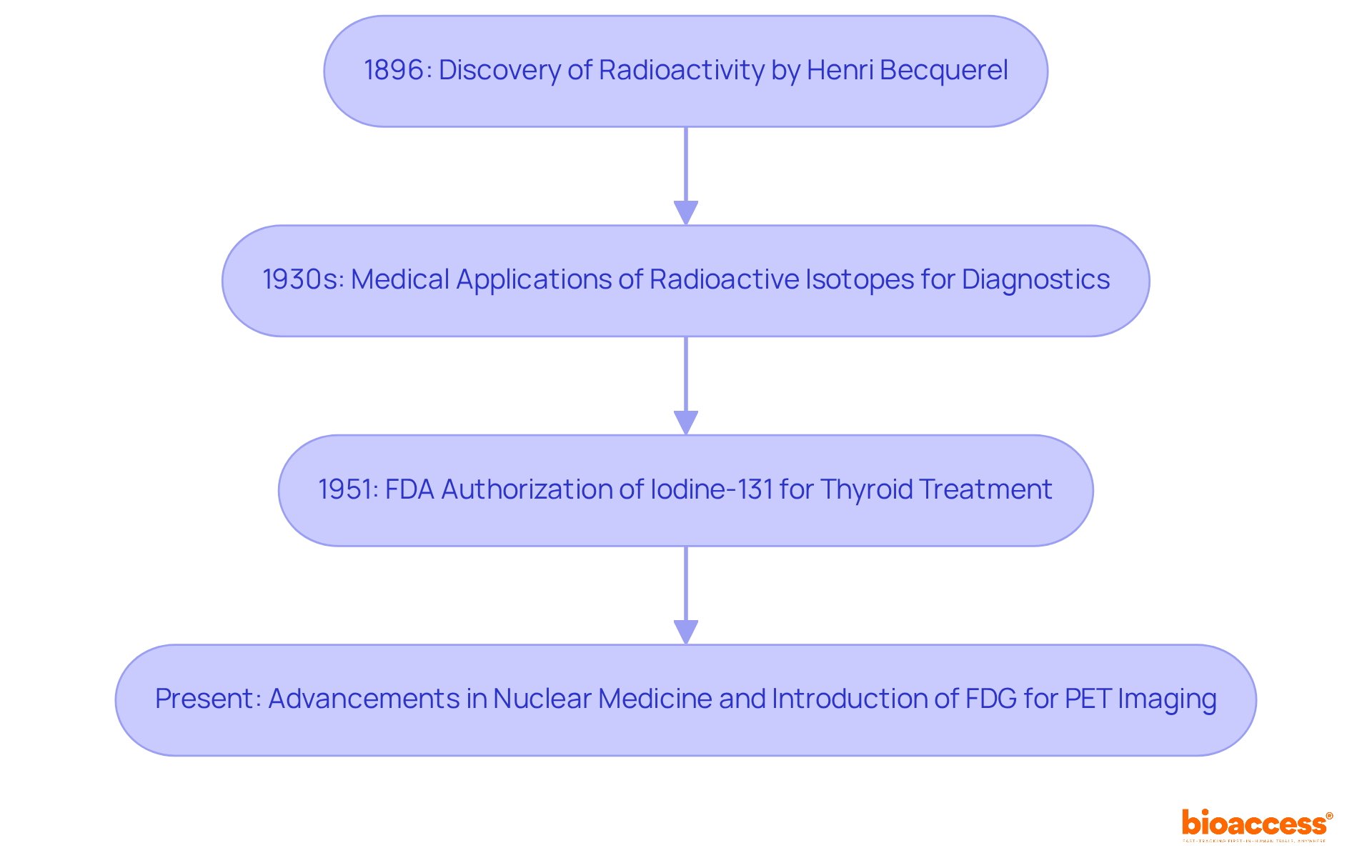
The history of radioactive drugs traces back to the early 20th century, initiated by Henri Becquerel's discovery of radioactivity in 1896. The medical application of radioactive isotopes first emerged in the 1930s, primarily aimed at diagnostic purposes. A pivotal moment occurred in 1951 when the FDA authorized iodine-131 for the treatment of thyroid disease, marking the inception of radioactive medicines as a viable therapeutic option.
Over the decades, advancements in nuclear medicine have catalyzed the development of various radiopharmaceuticals, with fluorodeoxyglucose (FDG) for PET imaging being a prominent radiopharmaceuticals example that has established itself as a standard in oncology. This historical perspective not only highlights the evolution of radioactive drugs but also underscores the relentless innovation in the field, driven by an ongoing demand for more effective diagnostic and therapeutic tools.
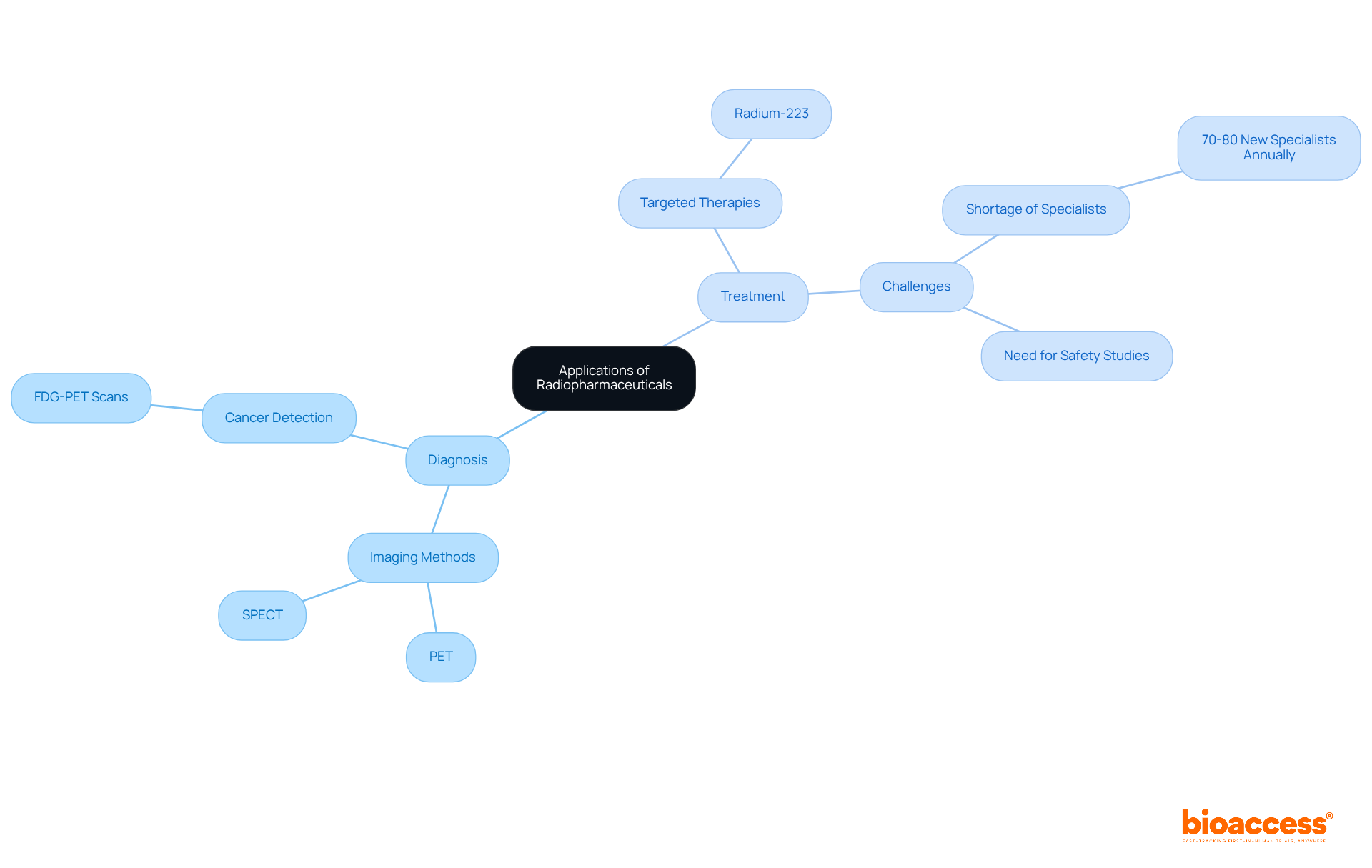
An example of radiopharmaceuticals is their crucial role in modern medicine, fulfilling both diagnostic and therapeutic functions. In diagnostics, a radiopharmaceuticals example is integral to imaging methods such as PET and SPECT, which are essential for identifying and monitoring various illnesses, including tumors, cardiovascular issues, and neurological conditions. For instance, FDG-PET scans serve as a radiopharmaceuticals example that are extensively utilized to evaluate metabolic activity in tumors, significantly aiding in diagnosis and therapeutic planning. On the therapeutic side, a radiopharmaceuticals example like radium-223 is specifically formulated to address metastatic prostate cancer by delivering targeted radiation to bone lesions, effectively minimizing damage to surrounding healthy tissues.
Nonetheless, the field encounters significant challenges, notably a shortage of trained nuclear medicine physicians, with only 70 to 80 new specialists entering the workforce each year in the US. This scarcity limits the potential for personalized therapies. Furthermore, there is an urgent need for comprehensive safety studies to monitor potential delayed effects stemming from radioactive drug therapies. Notably, around 50% of all cancer patients receive radiation therapy at some point during their treatment, underscoring the vital role of a radiopharmaceuticals example in enhancing diagnostic accuracy and treatment effectiveness.
Initiatives such as the Radiopharmaceutical Development Initiative (RDI) aim to bolster early-phase trials involving nuclear medicine, paving the way for future advancements in this field. With bioaccess®'s expert services, clinical trials can be accelerated, ensuring that innovative medical products are brought to market more swiftly, ultimately benefiting both patients and healthcare providers.
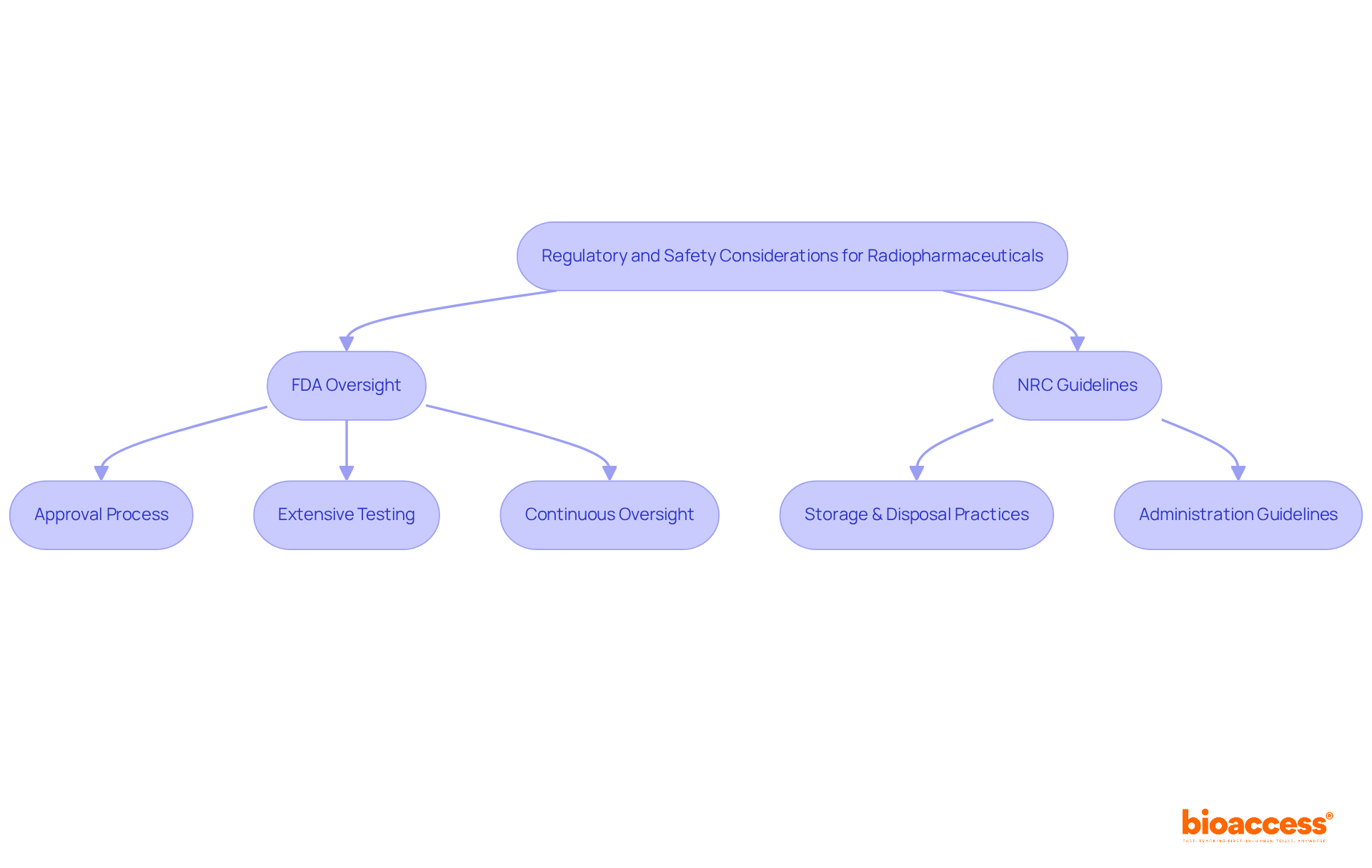
The oversight of radioactive medications is crucial for ensuring the safety of individuals and the effectiveness of treatment. In the United States, the Food and Drug Administration (FDA) plays a pivotal role in overseeing the approval and regulation of these substances, mandating extensive testing to confirm their safety and effectiveness prior to clinical use. Complementing this, the Nuclear Regulatory Commission (NRC) establishes stringent guidelines for the safe handling and administration of radioactive materials.
Essential safety protocols are designed to reduce radiation exposure for both individuals receiving care and healthcare providers. These protocols include:
Furthermore, the FDA's continuous oversight of authorized nuclear medicines guarantees adherence to safety regulations, reinforcing the commitment to safety for individuals in nuclear medicine.
Comprehensive clinical trial management services, such as those offered by bioaccess, are vital in navigating these regulatory frameworks. This encompasses:
These elements are essential for maintaining the integrity of clinical research and ensuring that a radiopharmaceuticals example is utilized safely and effectively in patient care.
Radiopharmaceuticals represent a groundbreaking advancement in modern medicine, integrating radioactive isotopes into therapeutic and diagnostic applications. Their ability to deliver targeted radiation and enhance imaging techniques has transformed the landscape of disease management, particularly in oncology, where precision is paramount. As the field continues to evolve, the significance of radiopharmaceuticals in improving patient outcomes cannot be overstated.
Key examples such as [Lu]Lu-PSMA-617 and [177Lu]Lu-DOTA-TATE illustrate the tangible benefits of these innovative therapies. The historical development of radiopharmaceuticals, beginning with early discoveries and leading to contemporary applications, highlights a trajectory of relentless innovation. Furthermore, the critical role of regulatory bodies like the FDA and NRC ensures that safety and efficacy remain at the forefront of clinical practices.
Ongoing challenges within the field, such as the shortage of trained professionals and the need for comprehensive safety studies, underscore the importance of continued research and development. By fostering collaboration and investing in initiatives like the Radiopharmaceutical Development Initiative, the medical community can pave the way for future breakthroughs. Embracing these advancements is essential not only for enhancing diagnostic accuracy and treatment effectiveness but also for ensuring that patients receive the best possible care in their health journeys.
What are radiopharmaceuticals?
Radiopharmaceuticals are specialized drugs that incorporate radioactive isotopes, playing a crucial role in both diagnostic and therapeutic applications in medicine.
Why are radiopharmaceuticals important in medicine?
They are significant in nuclear medicine due to their ability to deliver targeted radiation to specific tissues, which aids in accurate imaging and management of various medical conditions, particularly tumors.
How many radioactive pharmaceuticals are currently authorized globally?
There are 67 radioactive pharmaceuticals authorized globally, with 54 designated for diagnosis and 13 for treatment.
What medical conditions do radiopharmaceuticals address?
Radiopharmaceuticals address a range of conditions, including tumors, neurodegenerative disorders, and heart diseases.
What imaging techniques utilize radiopharmaceuticals?
Techniques such as Positron Emission Tomography (PET) and Single Photon Emission Computed Tomography (SPECT) leverage radiopharmaceuticals to produce detailed images of the body's internal structures.
What are some emerging trends in the use of radiopharmaceuticals as we approach 2025?
Emerging trends include the integration of radioactive drugs into clinical practice, combination therapies, and innovative agents aimed at improving treatment effectiveness and patient outcomes.
Can you provide examples of notable radiopharmaceuticals?
Notable examples include [Lu]Lu-PSMA-617, approved in 2022 for treating metastatic castration-resistant prostate conditions, and [177Lu]Lu-DOTA-TATE, approved in 2018 for neuroendocrine tumors.