


The article delineates a four-step process for effective post-market vigilance training in Mexico, underscoring the significance of:
Each step is bolstered by evidence indicating that structured training initiatives yield higher compliance rates, enhance patient safety, and boost organizational productivity and profitability. This reinforces the pivotal role of education in adeptly navigating regulatory landscapes.
Navigating the complex landscape of post-market vigilance training in Mexico necessitates a comprehensive understanding of regulatory frameworks and compliance requirements. With the evolving guidelines established by COFEPRIS, organizations possess a unique opportunity to enhance patient safety and operational efficiency through effective training programs. The challenge, however, persists: how can companies ensure that their training not only adheres to regulatory standards but also cultivates a culture of continuous improvement and stakeholder engagement? This article delineates four essential steps to develop and implement a robust post-market vigilance training program that addresses these critical concerns.
Navigating post-market vigilance training Mexico necessitates a comprehensive understanding of the regulations established by COFEPRIS (Comisión Federal para la Protección contra Riesgos Sanitarios). Central to this is adherence to NOM-240, which delineates the standards for technovigilance, including the protocols for adverse event reporting and monitoring device performance.
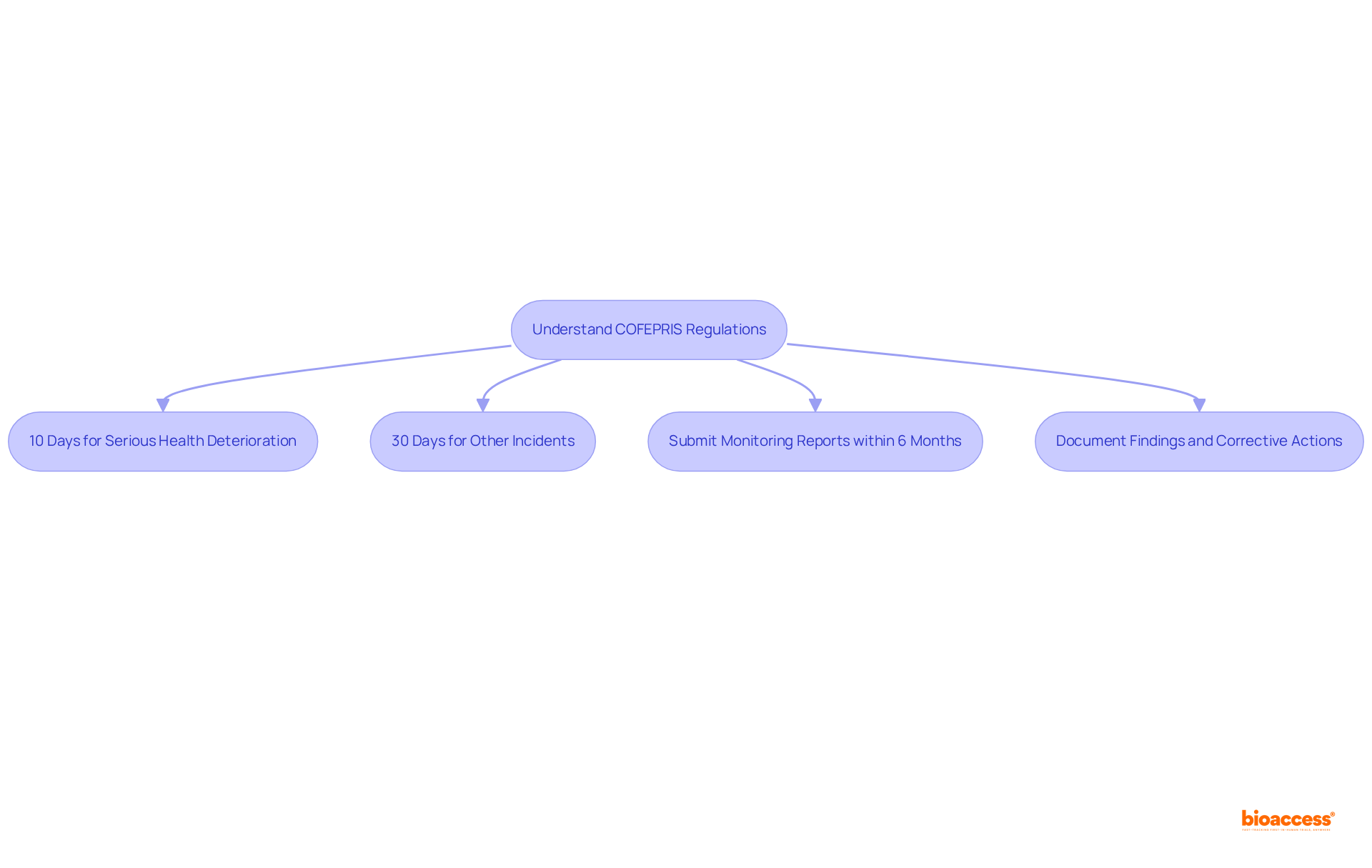
Organizations must remain informed of the latest COFEPRIS guidelines to ensure their educational programs align with current regulatory expectations. Adverse incidents must be reported within specific timelines:
Additionally, organizations are mandated to submit monitoring and final reports within six months unless an extension is granted. The implications of these requirements on operational processes are significant, as timely reporting and meticulous documentation are critical for upholding regulations and ensuring patient safety.
Establishing a technovigilance unit operated by knowledgeable personnel is essential for overseeing these activities. Furthermore, it is crucial to document findings and corrective actions for all incidents, even if they are not reported, to justify decisions later. By creating a robust development system that encompasses these components, organizations can effectively manage their obligations through post-market vigilance training Mexico.
It is also important to note that the new draft for NOM-240-SSA1-2024 is currently open for input and comments until September 29, 2024, which may further influence compliance requirements.
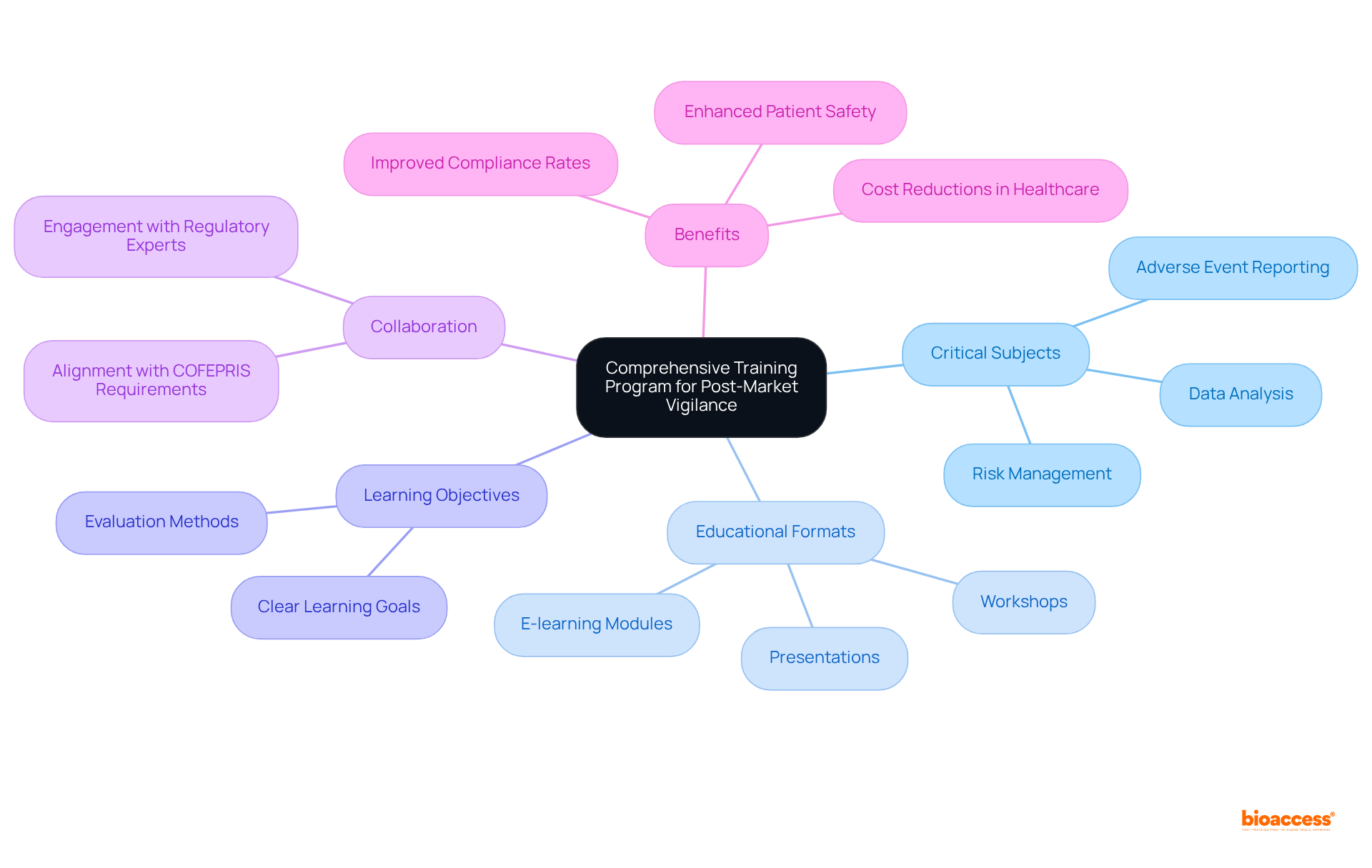
To establish a comprehensive educational course for post-market vigilance, it is imperative to identify the critical subjects that must be covered, including:
Engaging and informative educational materials should be developed, utilizing diverse formats such as:
Incorporating real-world scenarios and case studies is essential to deepen understanding. Additionally, it is crucial to set clear learning objectives and evaluation methods to assess the program's effectiveness. Collaborating with regulatory experts will ensure that the content aligns with COFEPRIS requirements and industry standards. Notably, businesses that lack organized development initiatives exhibit adherence rates around 60%, while those with systematic programs report adherence rates exceeding 85%. This underscores the importance of effective education in maintaining regulatory compliance and enhancing patient safety.
As Ana Criado stated, "Implementing an effective reporting system transcends mere regulatory compliance; it is a fundamental aspect of patient well-being and corporate integrity." Furthermore, investing in efficient educational initiatives can lead to a 23% reduction in healthcare costs associated with preventable adverse incidents, highlighting the financial benefits of prioritizing education. Additionally, advanced analytical tools have shown a significant decrease in adverse event reporting times, further emphasizing the critical role of data analysis in post-market vigilance training in Mexico.
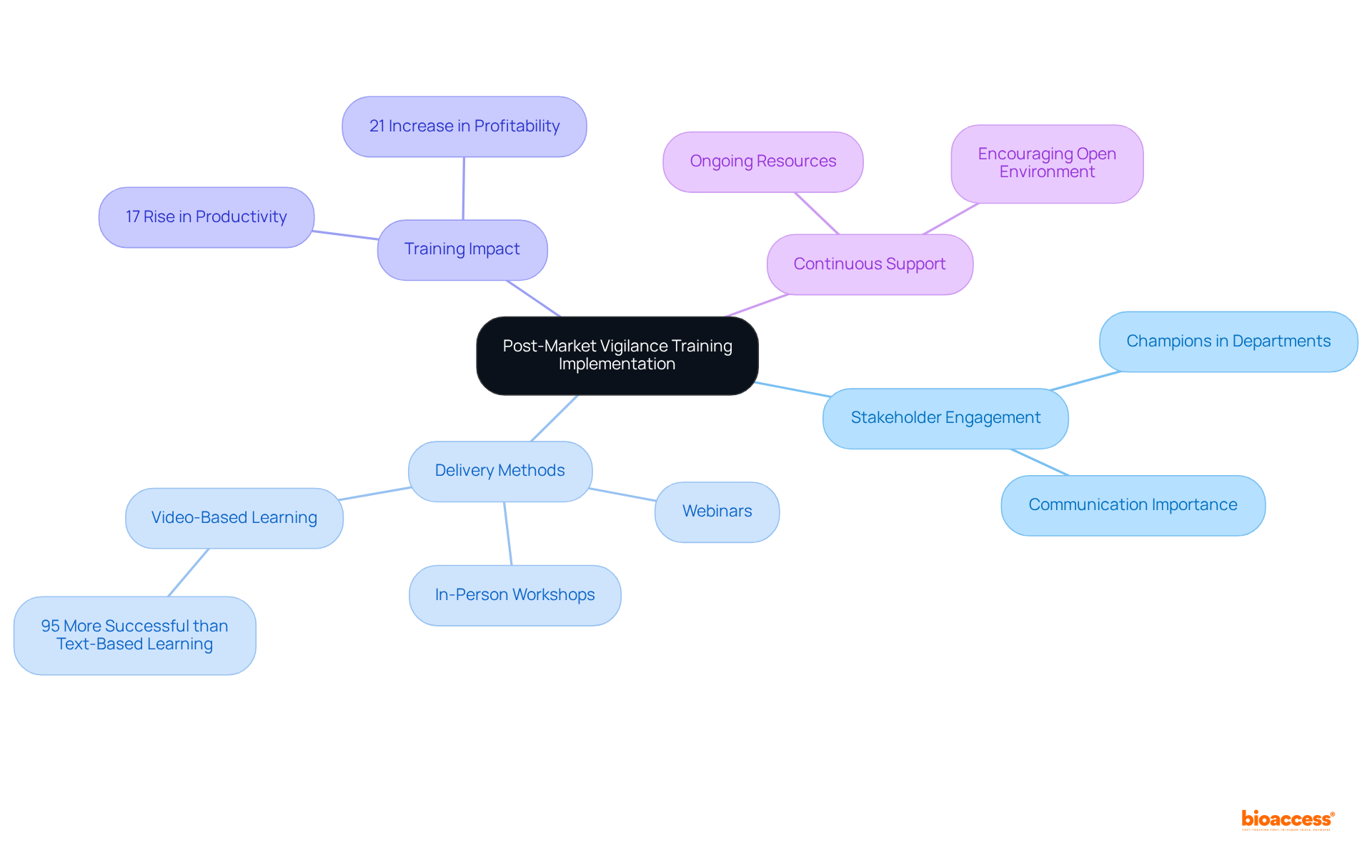
To effectively implement post-market vigilance training in Mexico, it is essential to schedule sessions that accommodate all relevant stakeholders, including regulatory affairs, quality assurance, and clinical teams. Employing a diverse range of delivery methods caters to various learning styles, such as in-person workshops, webinars, and interactive online courses. This approach not only enhances engagement but also aligns with the preferences of 68% of employees who favor learning at work. Notably, video-based learning proves to be 95% more successful than text-based learning, making it a valuable method to incorporate.
Fostering an open environment where questions and discussions are encouraged is crucial for participation. Regular communication about the significance of post-market vigilance training in Mexico—highlighting its direct impact on patient safety and product efficacy—reinforces the importance of this training. Furthermore, offering continuous assistance and resources enhances learning outcomes, as organizations that emphasize development experience a 17% rise in productivity and a 21% increase in profitability.
Consider designating champions in each department to promote development and support their peers. This strategy can significantly enhance stakeholder engagement, as companies that actively involve stakeholders are 30% more likely to succeed with new products. Moreover, businesses with extensive corporate development initiatives achieve 218% greater earnings per employee compared to those lacking structured instruction. By incorporating these best practices, organizations can establish a strong development initiative that not only fulfills compliance standards but also fosters a culture of ongoing enhancement and teamwork.
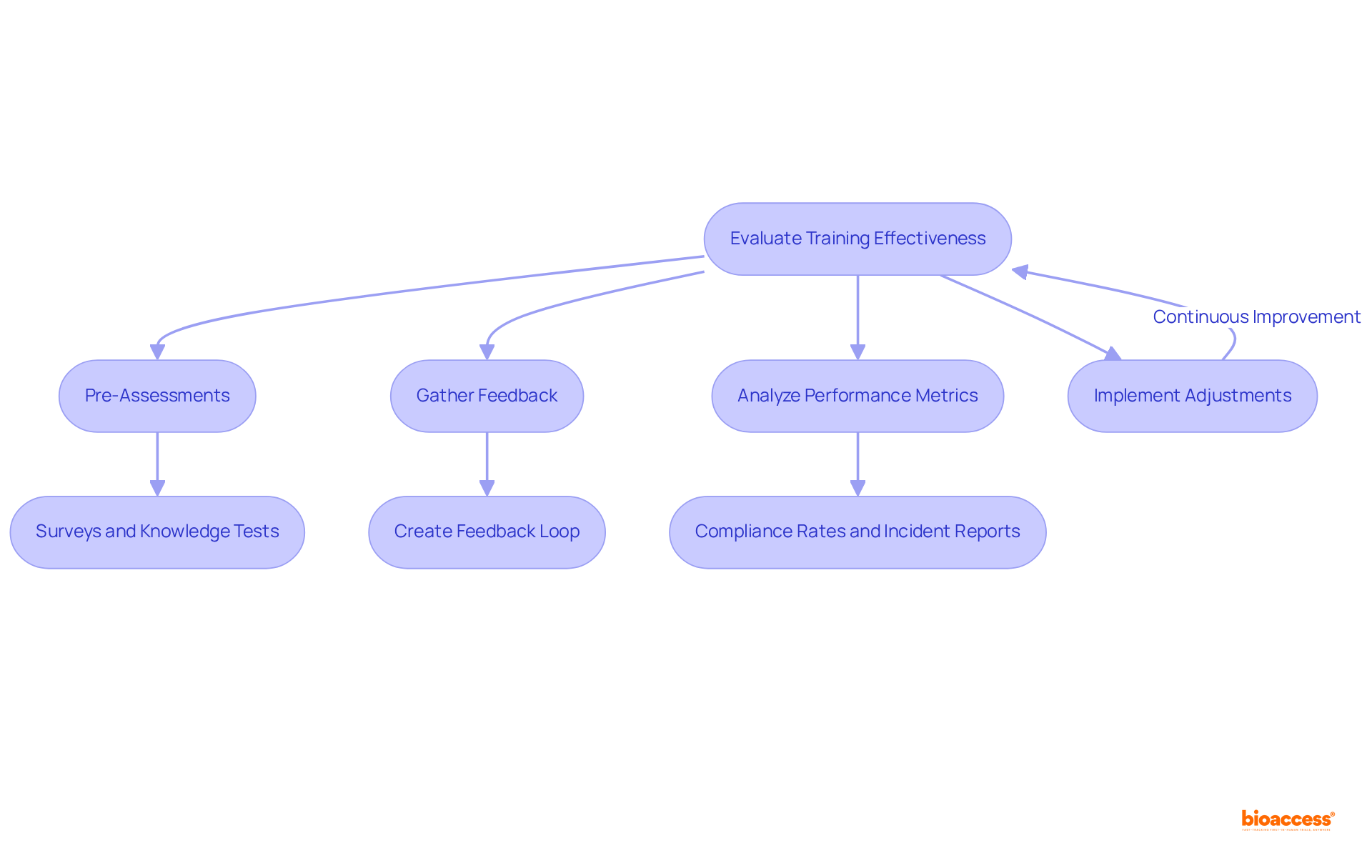
To effectively assess the program's impact, it is essential to employ a blend of pre- and post-assessments aimed at measuring knowledge retention and practical application. Preliminary evaluations, such as surveys and knowledge tests, reveal existing knowledge gaps, while post-instruction assessments measure the effectiveness of learning in real-world scenarios. Gathering participant feedback through surveys and interviews is crucial for pinpointing areas needing improvement.
Furthermore, examining performance metrics—such as compliance rates and incident reports—offers insights into how instruction affects post-market vigilance training in Mexico. Organizations that implement structured feedback mechanisms often see a 17% increase in productivity, underscoring the value of continuous improvement. Moreover, businesses with extensive staff development initiatives experience 218% greater revenue per employee than those lacking such initiatives, highlighting the economic advantages of efficient evaluation methods.
Based on these evaluations, necessary adjustments should be made to the development content, delivery methods, and support resources. Creating a continuous feedback loop ensures that the program remains aligned with evolving regulations and industry best practices, ultimately enhancing its effectiveness and relevance. As Shani Jay notes, measuring training effectiveness should be a continuous process, enabling employees to feel supported and empowered at work.
The significance of effective post-market vigilance training in Mexico cannot be overstated. Adhering to the regulatory standards set forth by COFEPRIS and implementing a structured training program allows organizations not only to ensure compliance but also to enhance patient safety and operational efficiency. The journey toward excellence in post-market vigilance begins with a solid understanding of regulations, followed by the development and implementation of comprehensive training programs that engage all stakeholders involved.
Key steps have been outlined to facilitate successful post-market vigilance training. These include:
Each of these components plays a crucial role in reinforcing knowledge retention and practical application, ultimately leading to improved compliance rates and reduced adverse incidents.
Organizations must recognize that investing in robust post-market vigilance training is not merely a regulatory obligation but a vital aspect of corporate integrity and patient well-being. By prioritizing education and fostering a culture of continuous improvement, businesses can significantly enhance their operational outcomes and contribute to safer healthcare environments. Embracing these best practices ensures that organizations are well-equipped to navigate the evolving landscape of post-market vigilance in Mexico, ultimately benefiting patients and the healthcare system as a whole.
What is the main regulatory body for post-market vigilance training in Mexico?
The main regulatory body is COFEPRIS (Comisión Federal para la Protección contra Riesgos Sanitarios).
What standards must organizations adhere to for technovigilance in Mexico?
Organizations must adhere to NOM-240, which outlines the standards for technovigilance, including protocols for adverse event reporting and monitoring device performance.
What are the reporting timelines for adverse incidents in Mexico?
Serious health deterioration must be reported within 10 days, while other incidents must be reported within 30 days.
What is the deadline for submitting monitoring and final reports?
Monitoring and final reports must be submitted within six months, unless an extension is granted.
Why is timely reporting and documentation critical in post-market vigilance?
Timely reporting and meticulous documentation are critical for upholding regulations and ensuring patient safety.
What is essential for overseeing post-market vigilance activities in organizations?
Establishing a technovigilance unit operated by knowledgeable personnel is essential for overseeing these activities.
What should organizations document regarding adverse incidents?
Organizations should document findings and corrective actions for all incidents, even if they are not reported, to justify decisions later.
What is the significance of the new draft for NOM-240-SSA1-2024?
The new draft is open for input and comments until September 29, 2024, and may further influence compliance requirements for organizations.