


The article delineates a four-step process for obtaining export certificates in Mexico, underscoring the critical importance of compliance to ensure smooth customs clearance for a variety of products. It elucidates specific documentation requirements, addresses common challenges faced by exporters, and examines the evolving regulatory landscape. Together, these elements highlight the necessity of thorough preparation and a comprehensive understanding of the export certificate issuance process.
Navigating the intricate world of international trade demands a solid understanding of compliance, particularly concerning export certificates in Mexico. These essential documents not only confirm adherence to regulations but also facilitate the smooth movement of goods across borders. As exporters confront increasingly complex requirements and evolving regulations, the challenge is to comprehend the specific certifications necessary for various products.
How can businesses effectively streamline the export certificate issuance process to prevent costly delays and ensure successful market entry?
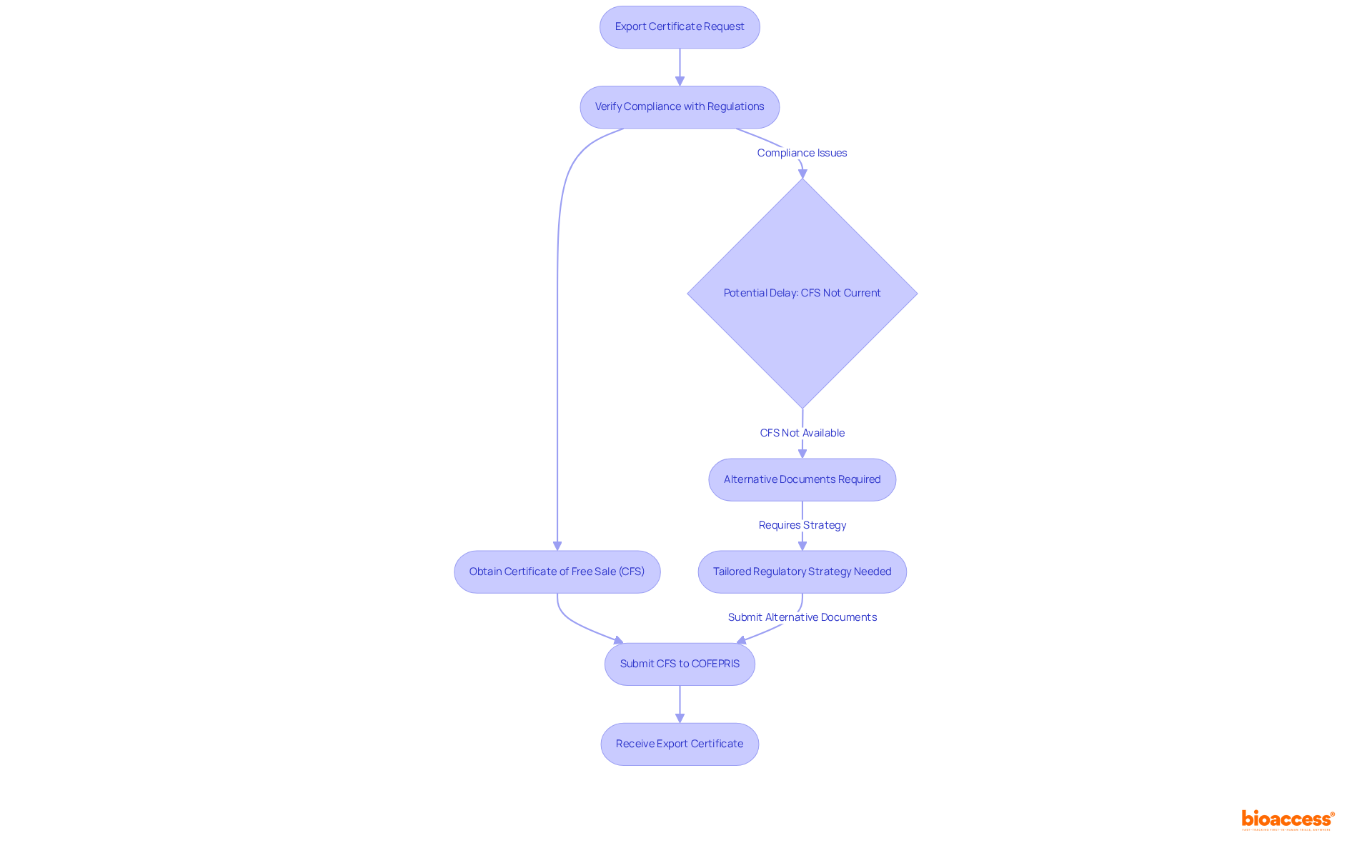
The export certificate issuance in Mexico helps verify that export documents are crucial papers confirming an item's compliance with regulations, particularly for medical devices, pharmaceuticals, and food items. These documents transcend mere bureaucratic requirements; they are crucial for export certificate issuance Mexico help, ensuring that products can navigate customs seamlessly. Without the appropriate export certificate issuance, Mexico help may be necessary to prevent significant delays or denial of entry for items, which can result in increased costs and lost opportunities for sellers.
For instance, the export certificate issuance in Mexico can help secure the Certificate of Free Sale (CFS), which is vital for obtaining Sanitary Registration for foreign-manufactured medical devices. This document must be issued by the Ministry of Health in the country of origin and must provide details about the product, including its specific name and manufacturing location. In the absence of a CFS, alternative documents may be accepted, but this often requires a tailored regulatory strategy, complicating the shipping process.
Recent changes in shipping documentation regulations have further underscored the need for compliance. Exporters are now required to ensure that their CFS is no older than 30 months at the time of submission to COFEPRIS, the Mexican regulatory authority. This stipulation highlights the importance of keeping documentation current to help with export certificate issuance in Mexico and prevent customs delays. Additionally, the one-year pause instituted by FAS Bogota on Decree 2478 emphasizes the evolving landscape of shipping regulations that sellers must navigate.
Real-world scenarios illustrate the challenges exporters confront. Many have encountered compliance issues due to incomplete or outdated shipping certificates, but export certificate issuance Mexico help can alleviate these problems and prevent significant customs delays. Such situations demonstrate the necessity of understanding the specific requirements for each product category, including export certificate issuance Mexico help, to facilitate smooth entry into the Mexican market. As Steve Pniewski, Founder & CEO of Artemus Transportation Solutions, notes, "Understanding how to send medicine from the USA is a complex and highly regulated process, involving adherence to stringent federal laws, documentation, and compliance with international trade requirements."
In conclusion, the importance of shipment documents cannot be overstated. They are vital for navigating the complexities of customs clearance in Mexico, and their absence can result in costly delays and compliance challenges. As the trade regulation landscape continues to evolve, it is essential to remain informed and prepared for successful market entry.
To successfully ship items to Mexico, the export certificate issuance Mexico help is crucial in determining the specific documentation required for your goods. The common types of export certificates include:
In addition to understanding these document types, it is essential to consider the statistics related to shipping document requirements for pharmaceuticals in Mexico. For instance, the value of pharmaceutical shipments from Mexico has been monitored from 2014 to 2023, indicating significant growth potential in this sector. Furthermore, the CDRH Export Document Validation database serves as a crucial tool for confirming documents issued prior to January 2, 2024, and will continue to operate until January 2, 2026.
With the FDA's transition to digital shipping documents commencing in December 2023, all request forms finalized after January 2 will be provided electronically as downloadable PDFs. The costs associated with acquiring trade documents are also noteworthy: the initial trade document issued from CDRH costs $175.00, while each additional document from the same request is $85.00.
To ensure compliance, it is advisable to research the specific requirements for your product category through resources such as the USDA or Mexican customs websites. Moreover, confirming that the accurate country-required statements are present on the shipping document is essential for regulatory compliance. Understanding these components will help with export certificate issuance in Mexico, facilitating smoother shipping procedures and enhancing your ability to effectively navigate the regulatory environment.
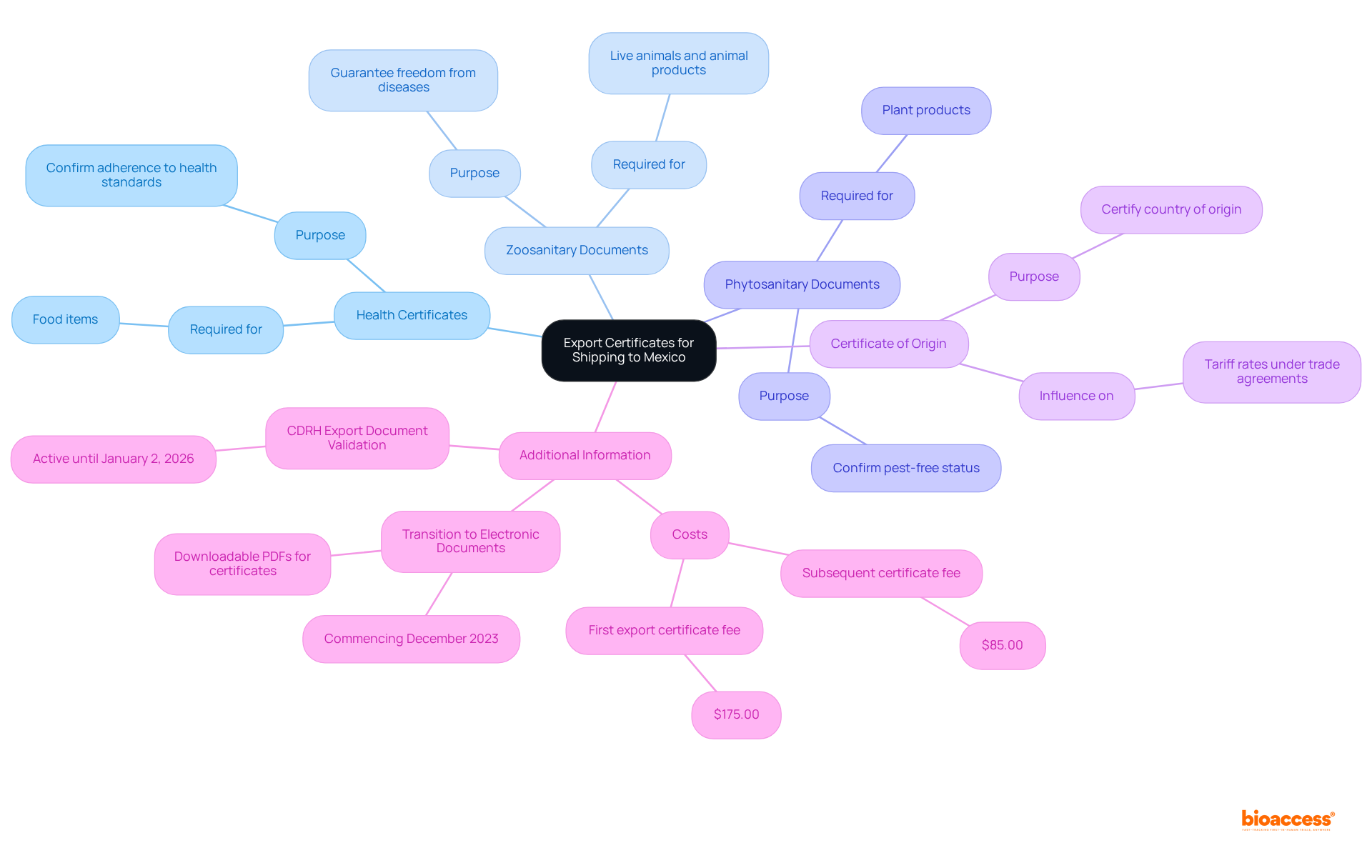
To apply for export certificates in Mexico, follow these essential steps:
Gather Required Documentation: Assemble all necessary documents, including product specifications, previous certifications, and relevant test results. This foundational step ensures that your submission, with the export certificate issuance Mexico help, is comprehensive and meets regulatory standards.
Complete the Form: Accurately fill out the specific form for the export certificate you require. Precision in this step is crucial; incomplete or incorrect information can lead to significant delays in the export certificate issuance Mexico help process.
Submit the Request: Forward your request along with the required documentation to the appropriate authority. For food products, this typically involves the USDA, while other goods may require the export certificate issuance Mexico help by submitting to a relevant Mexican agency.
Pay Any Fees: Be prepared to pay applicable fees related to your submission. These fees can differ depending on the type of document and the issuing authority, so it is wise to verify the most recent fee schedule, especially for export certificate issuance Mexico help.
Await Approval: After submission, keep track of your status. The processing duration for certification applications can vary from several days to weeks, and understanding the export certificate issuance in Mexico can help, as it is influenced by the complexity of the request and the specific type of certificate.
Recent updates indicate that as of June 3, 2025, Mexico has implemented new regulations requiring automatic shipment notifications for certain goods, including medical instruments. Notifications must be submitted starting June 30, 2025, and the Ministry of Economy will issue the notification within 10 business days after the request is submitted. This change underscores the importance of staying informed about evolving requirements to ensure compliance and facilitate more seamless shipping procedures.
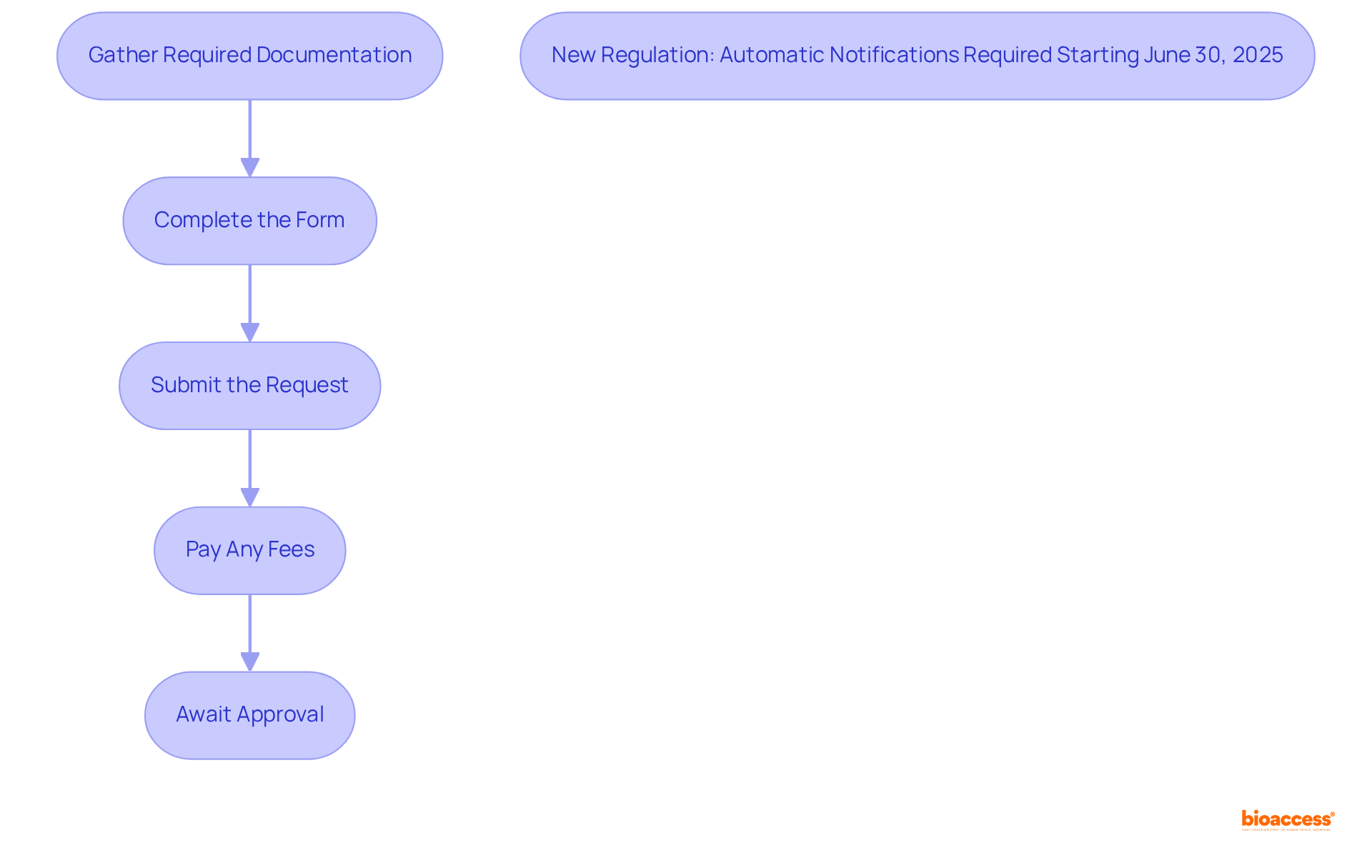
Exporters frequently encounter various challenges related to export certificate issuance in Mexico. Here are essential troubleshooting tips to navigate the process effectively:
Complete Forms: Ensure that every section of the form is thoroughly filled out. Incomplete submissions are a primary reason for delays and rejections, with statistics indicating that a substantial percentage of requests are returned due to missing information. For instance, submitting an export license request with mistakes can lead to it being flagged as 'Returned without Action' (RWA).
Correct Documentation: Verify that you are submitting the appropriate documents for your specific product type. Adhering to the guidelines set by the issuing authority is crucial to avoid unnecessary complications.
Processing Delays: If your request is taking longer than expected, proactively reach out to the issuing authority for an update. Delays may occur if additional information is required, and timely communication can help expedite the process.
Handling Rejections: In the event of a rejection, carefully analyze the feedback provided. Address the highlighted concerns and resend your submission at your earliest convenience to help with the export certificate issuance in Mexico and reduce holdups in your shipping strategies. The U.S. Bureau of Industry and Security notes that end-use statement errors are a major cause of approval delays, so ensure these are accurately completed.
Regulatory Changes: Stay informed about any modifications in export rules or requirements that could affect your submission. Regularly consult official websites or engage with a customs broker to ensure compliance with the latest standards. Additionally, understanding common problems faced during applications, such as incomplete information or poorly compiled end-use documentation, can help you avoid pitfalls.
Understanding the intricacies of export certificate issuance in Mexico is essential for businesses aiming to successfully navigate the complexities of international trade. These certificates serve as vital documents that confirm compliance with regulatory standards, particularly for sensitive products like medical devices, pharmaceuticals, and food items. The significance of ensuring timely and accurate export certificate issuance cannot be overstated, as it directly impacts a company's ability to enter the Mexican market without facing unnecessary delays or compliance issues.
Throughout the article, key points have been highlighted, including:
Specific insights into the importance of maintaining current documentation and understanding the evolving regulatory landscape have been emphasized, showcasing the need for exporters to remain vigilant and informed. Moreover, practical troubleshooting tips provide actionable steps to mitigate potential issues during the application process, reinforcing the idea that preparation is crucial for success.
Ultimately, the journey of exporting goods to Mexico can be complex and fraught with obstacles. However, by prioritizing the understanding of export certificate requirements and adhering to best practices, businesses can enhance their chances of successful market entry. Embracing this knowledge not only facilitates smoother shipping procedures but also positions exporters to seize valuable opportunities in the growing Mexican market. Taking proactive steps today ensures that businesses are well-equipped to thrive in an increasingly competitive global trade environment.
What is the significance of export certificates in Mexico?
Export certificates are crucial documents that verify an item's compliance with regulations, particularly for medical devices, pharmaceuticals, and food items. They facilitate seamless navigation through customs and help prevent delays or denial of entry for products.
What is the Certificate of Free Sale (CFS)?
The Certificate of Free Sale (CFS) is a vital document for obtaining Sanitary Registration for foreign-manufactured medical devices. It must be issued by the Ministry of Health in the country of origin and includes details about the product, such as its specific name and manufacturing location.
What happens if a CFS is not available?
If a CFS is not available, alternative documents may be accepted, but this often requires a tailored regulatory strategy, complicating the shipping process.
What recent changes have been made regarding shipping documentation regulations?
Exporters are now required to ensure that their CFS is no older than 30 months at the time of submission to COFEPRIS, the Mexican regulatory authority, emphasizing the need for current documentation to prevent customs delays.
What is the impact of the one-year pause instituted by FAS Bogota on Decree 2478?
The one-year pause emphasizes the evolving landscape of shipping regulations that sellers must navigate, highlighting the importance of compliance in the export process.
What challenges do exporters face regarding compliance?
Exporters often encounter compliance issues due to incomplete or outdated shipping certificates, which can lead to significant customs delays.
Why is it important to understand specific requirements for product categories?
Understanding the specific requirements for each product category is essential to facilitate smooth entry into the Mexican market and to navigate the complexities of customs clearance.
What should exporters do to prepare for successful market entry in Mexico?
Exporters should remain informed about the evolving trade regulation landscape and ensure that all necessary shipment documents are current and compliant to avoid costly delays and challenges.