


The article primarily addresses the essential steps necessary for achieving success in the technical file re-format service in Brazil, with a particular emphasis on compliance with ANVISA regulations. It delineates four critical steps:
Each of these steps is vital for ensuring timely approvals and minimizing non-compliance risks throughout the submission process.
Navigating the intricate landscape of Brazilian regulatory requirements presents a formidable challenge for companies striving to ensure compliance in their technical file submissions. Recent updates, including RDC 751/2022, underscore the necessity of grasping the nuances of these regulations for achieving success. This article delineates essential steps and strategies that can significantly streamline the technical file re-formatting process, thereby enhancing both efficiency and accuracy.
How can organizations effectively harness local expertise and technology to not only meet but exceed compliance expectations in Brazil?
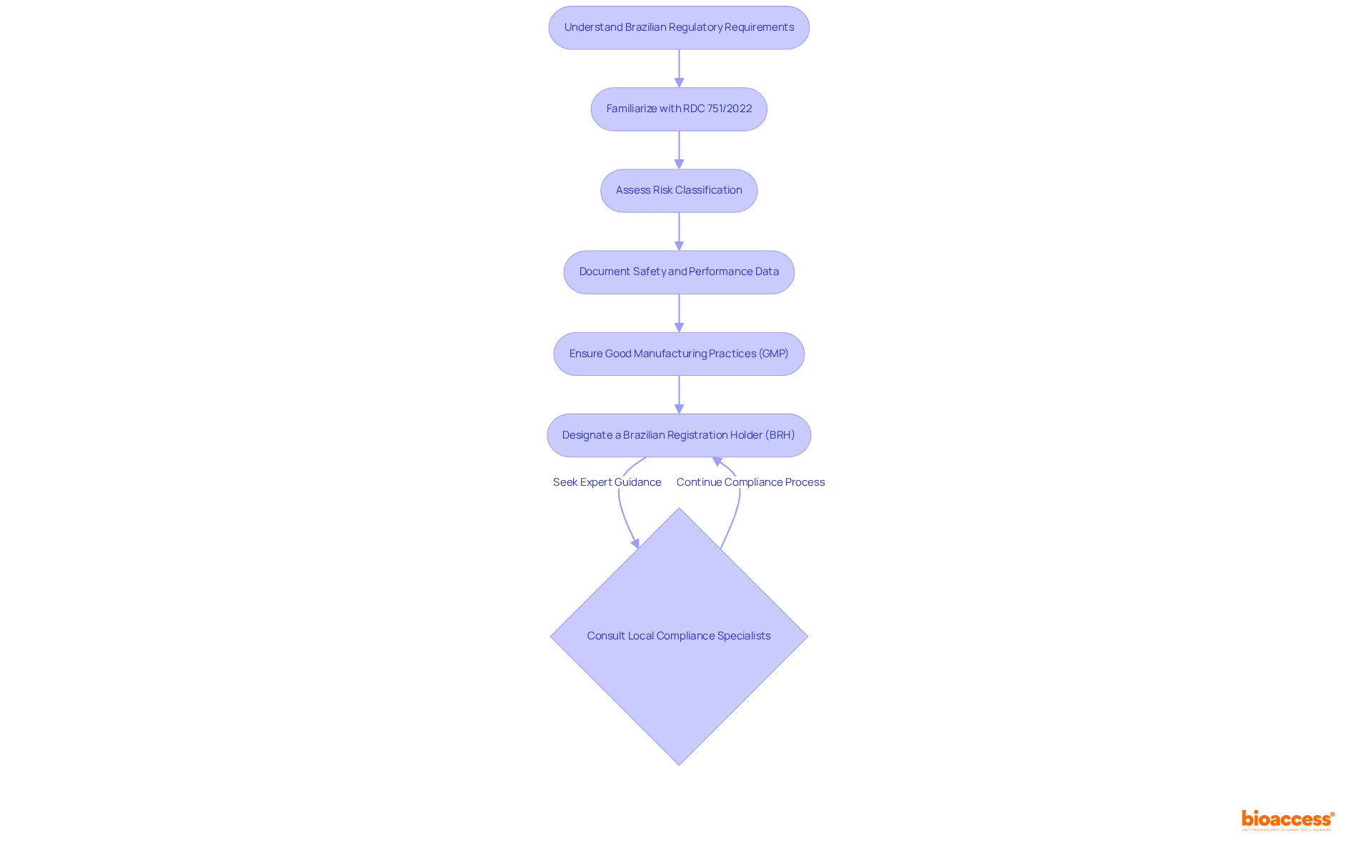
To successfully navigate the Brazilian regulatory environment, it is essential to familiarize yourself with the specific requirements established by the National Health Surveillance Agency. This includes understanding the latest regulations, such as RDC 751/2022, which outlines the structure and content necessary for the technical file re-format Brazil service. Key components include:
Furthermore, designating a Brazilian Registration Holder (BRH) is crucial, as they play an important role in the approval process and communication with the relevant authority. Consistently refer to sources such as the ANVISA site and industry news to remain aware of any alterations in compliance expectations.
Interacting with local compliance specialists, such as Ana Criado, Director of Compliance Affairs and CEO of Mahu Pharma, can provide invaluable insights into the nuances of adherence, ensuring that your technical files are complete and aligned with current standards, particularly when utilizing the technical file re-format Brazil service. Ana's extensive background in biomedical engineering and health economics, combined with her experience as a compliance consultant for global companies, underscores the significance of expert guidance in this process.
Bioaccess provides specialized services, including a technical file re-format Brazil service, to help navigate these regulatory requirements and ensure your submissions meet all necessary standards. Maintaining an updated documentation file is essential for notification and inspection by the National System of Health Surveillance. Prompt submissions of regular safety updates can enhance adherence rates by as much as 20%, helping to avoid typical challenges in compliance.
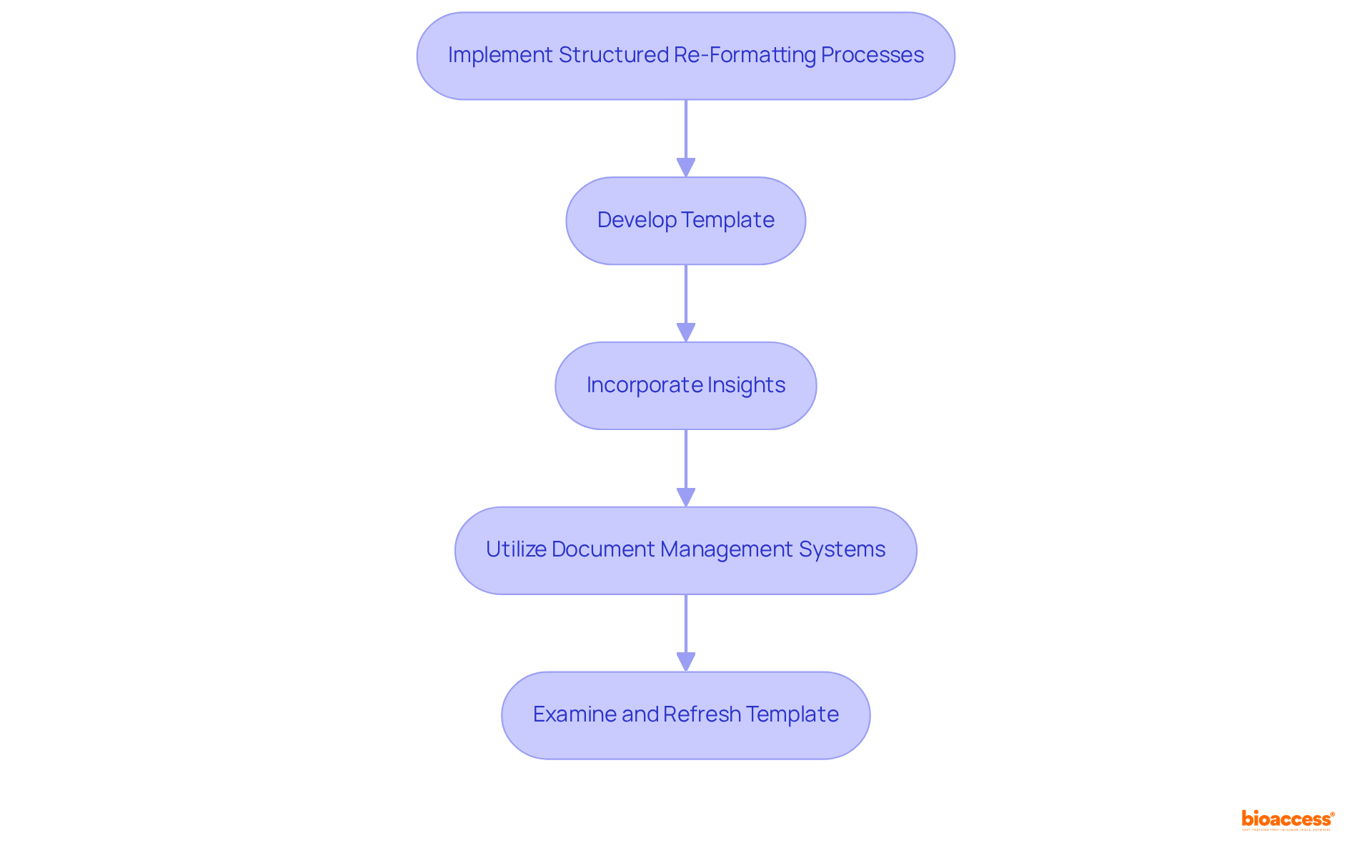
To successfully implement a structured process for the technical file re-format Brazil service, it is essential to break down the document into clearly defined sections that align with ANVISA's guidelines, particularly following the recent changes from RDC 185/2001 to RDC 751/2022.
Begin by developing a comprehensive template that encompasses all necessary chapters, including:
Integrate insights from bioaccess's extensive service capabilities—such as:
to ensure thoroughness and adherence to standards.
Utilize document management systems to ensure version control and enhance collaboration among team members. Consistently examine and refresh the template to include any alterations in compliance requirements.
This systematic method not only streamlines the technical file re-format Brazil service but also significantly minimizes the risk of non-compliance, ultimately enabling approvals that can be 50% quicker than those in traditional markets.
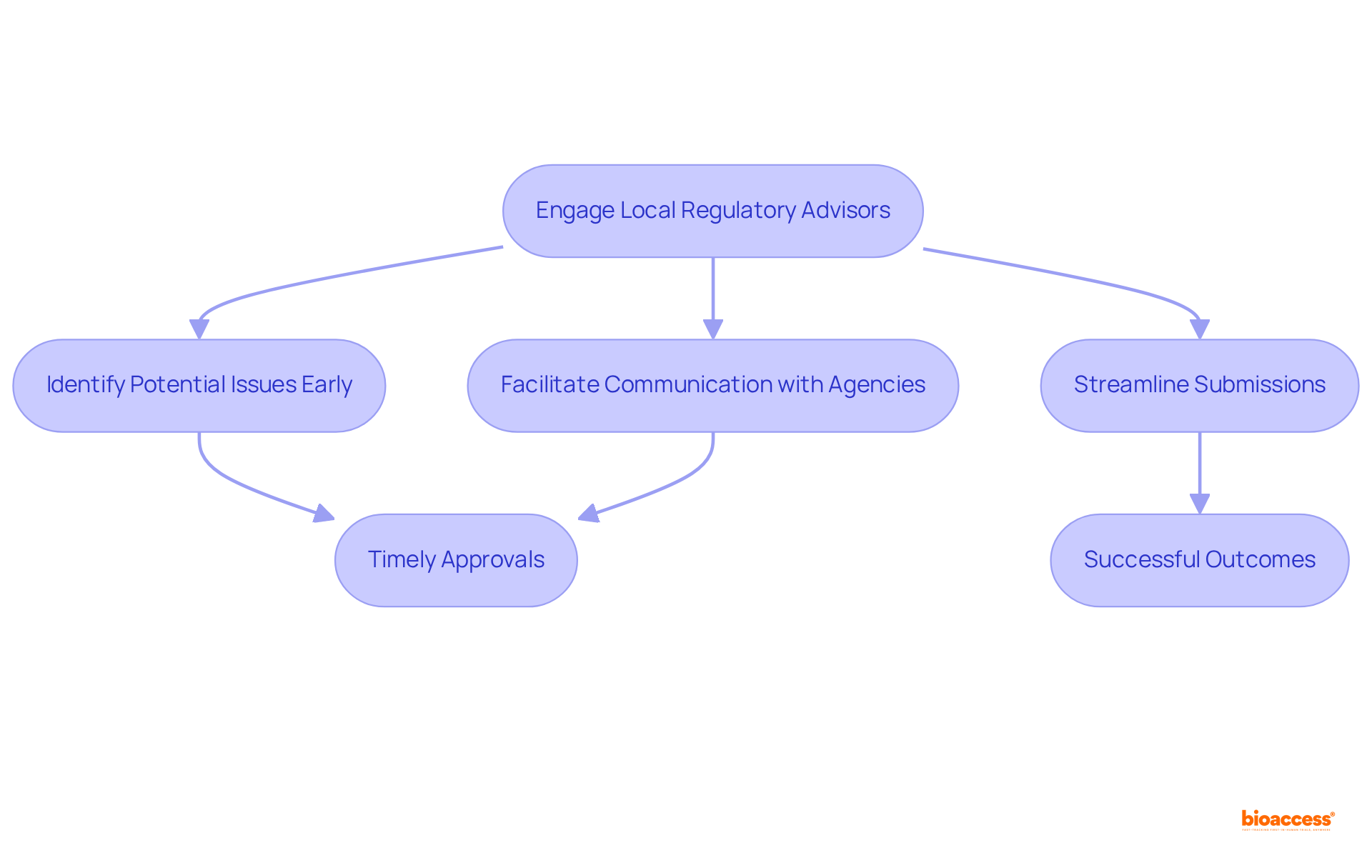
Engaging local regulatory advisors significantly enhances the effectiveness of the technical file re-format Brazil service for submissions to the agency. These professionals possess comprehensive knowledge of ANVISA's requirements and provide invaluable insights into best practices for documentation. Their expertise allows them to identify potential issues early, ensuring that all necessary information is meticulously included in the documentation.
Furthermore, local specialists facilitate effective communication with governing agencies, addressing any questions or concerns that may arise during the review process. By leveraging their specialized knowledge, companies can streamline their submissions for the technical file re-format Brazil service, which increases the likelihood of timely approvals and successful outcomes.
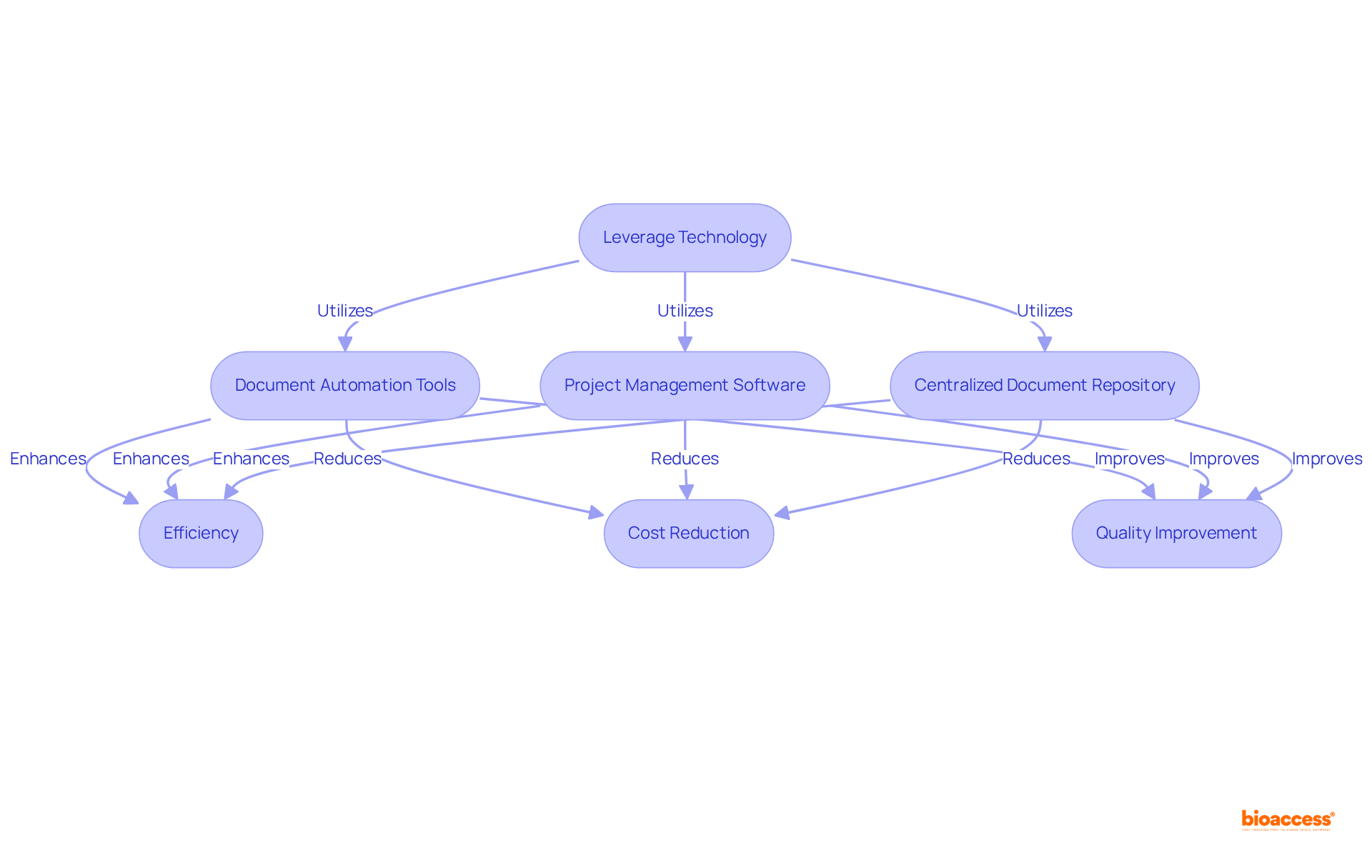
Utilizing technology for the technical file re-format Brazil service significantly enhances both efficiency and accuracy, particularly in the realm of clinical research. Document automation tools play a vital role in standardizing formatting and ensuring adherence to requirements, particularly in the technical file re-format Brazil service, especially with ANVISA standards. These tools can reduce operational expenses by up to 50% and shorten cycle durations by as much as 85%, making them essential for regulatory submissions.
Furthermore, project management software fosters collaboration among team members, enabling real-time updates and feedback—crucial elements for maintaining compliance. Implementing a centralized document repository guarantees that all stakeholders have access to the most current versions of technical files, further streamlining the submission process.
By adopting these technological solutions, organizations can minimize the time spent on re-formatting their technical file re-format Brazil service while significantly improving the quality of their submissions.
Successfully navigating the Brazilian regulatory landscape for technical file re-formatting is crucial for organizations aiming to ensure compliance and efficiency. Understanding the specific requirements set forth by ANVISA, implementing structured re-formatting processes, engaging local experts, and leveraging technology significantly enhances the chances of achieving timely approvals and successful outcomes.
Key insights emphasize the importance of:
In light of these strategies, organizations are encouraged to take proactive steps towards mastering the technical file re-format Brazil service. By prioritizing regulatory compliance and embracing best practices, businesses position themselves for success in the competitive landscape of medical device regulation in Brazil. Engaging with experts and adopting innovative solutions will enhance compliance and foster a culture of continuous improvement, ensuring that organizations remain at the forefront of regulatory excellence.
What are the key components of the technical file required for Brazil?
The key components include risk classification, documentation of safety and performance data, and adherence to Good Manufacturing Practices (GMP).
What is the significance of the Brazilian Registration Holder (BRH)?
The Brazilian Registration Holder (BRH) is crucial as they play an important role in the approval process and communication with the National Health Surveillance Agency (ANVISA).
How can one stay updated on Brazilian regulatory requirements?
It is important to consistently refer to sources such as the ANVISA site and industry news to remain aware of any alterations in compliance expectations.
Who can provide expert guidance on compliance with Brazilian regulatory requirements?
Local compliance specialists, such as Ana Criado, Director of Compliance Affairs and CEO of Mahu Pharma, can provide invaluable insights into adherence nuances.
What services does Bioaccess offer related to Brazilian regulatory compliance?
Bioaccess provides specialized services, including a technical file re-format Brazil service, to help navigate regulatory requirements and ensure submissions meet necessary standards.
Why is maintaining an updated documentation file important?
Maintaining an updated documentation file is essential for notification and inspection by the National System of Health Surveillance.
How can timely submissions of safety updates impact compliance?
Prompt submissions of regular safety updates can enhance adherence rates by as much as 20%, helping to avoid typical challenges in compliance.