


The article outlines four essential steps for designing a successful placebo control study:
These steps are crucial as they ensure the study is methodologically sound, ethically compliant, and capable of yielding reliable results. The emphasis on randomization, blinding, and ethical considerations throughout the research design process reinforces the necessity of these components, ultimately contributing to the integrity and credibility of clinical research.
Designing a successful placebo control study is a fundamental aspect of clinical research that can significantly influence the validity of treatment evaluations. By carefully orchestrating the balance between active treatment and placebo, researchers can uncover the true efficacy of new therapies while navigating complex ethical considerations.
However, with the potential for bias and the challenge of participant expectations, how can one ensure that the study design is robust and reliable? This guide delves into the essential steps and methodologies required to create a well-structured placebo control study, equipping researchers with the tools they need to generate trustworthy results.
A placebo control study is a critical research design in which one group of individuals receives the active treatment while another group is administered a placebo—an inactive substance that mimics the treatment. This methodology is essential for evaluating the efficacy of the treatment by juxtaposing outcomes between the two groups. The placebo effect, where individuals experience perceived improvements due to their expectations, can significantly sway study results. Notably, research indicates that approximately 35% of patients may respond positively to placebo treatment, highlighting its potential influence on clinical outcomes.
Grasping key methodologies such as randomization and blinding is crucial in this context. Randomization ensures that individuals are allocated to treatment groups in a manner that minimizes bias, while blinding—where individuals and/or researchers remain unaware of group assignments—further bolsters the reliability of findings. For instance, in a recent double-blind, placebo control study assessing an Ayurvedic formulation for stress relief, significant reductions in anxiety levels were observed in individuals receiving the active treatment compared to those on the placebo. This underscores the importance of rigorous research design.
Moreover, ethical considerations are paramount in the application of placebos. The Office for Human Research Protection emphasizes that continued assignment to placebo becomes unethical once there is evidence supporting the effectiveness of the experimental therapy. Consequently, informed consent forms must explicitly communicate the possibility of receiving a placebo, ensuring that participants are fully cognizant of the implications. It is also vital to recognize that placebos should not be utilized when this entails withholding effective treatment for serious and life-threatening conditions.
Successful examples of placebo control studies in the Medtech sector illustrate the efficacy of this design. The Novavax COVID-19 vaccine study, for example, utilized a robust placebo control study, which contributed to its eventual authorization and recognition as a significant advancement in vaccination efforts. By understanding the intricacies of placebo-controlled experiments, investigators can refine the design of tests that yield significant and trustworthy outcomes. At bioaccess®, we leverage over 20 years of expertise in managing clinical trials, including Early-Feasibility, First-In-Human, and Post-Market Follow-Up Studies, ensuring our clients navigate the complexities of clinical research with confidence.

Define Your Research Question: Clearly articulate the hypothesis you aim to test. This foundational step shapes the entire design and direction of your research. As Tala Harake emphasizes, "Make sure that you completely iron out the research question you are trying to answer, and have your protocol flow to answer that question."
Select Your Population: Identify a target population that accurately reflects the broader group affected by the condition. This selection is crucial for ensuring the generalizability of your findings. Meri Beckwith notes that "a well-defined population is critical, as it encompasses the entire group of individuals that the research aims to understand." In a placebo control study, it is essential to consider demographic factors like age, gender, and comorbidities to create a representative sample.
Determine Sample Size: Calculate the necessary number of subjects to achieve statistically significant results. This calculation should consider the anticipated effect size and the desired power of the research, usually targeting a power of at least 80% to identify significant differences. As Meri Beckwith states, "The selection of sample size is particularly crucial. Researchers often conduct power analyses to determine the minimum sample size needed to detect an effect if one exists."
In a placebo control study, implementing randomization and blinding helps assign participants to either the treatment or placebo group, which mitigates selection bias. Furthermore, utilizing blinding methods avoids bias in treatment delivery and outcome evaluation, improving the reliability of the research.
Develop Protocols: Create comprehensive protocols that detail the study procedures, including recruitment strategies, treatment administration, and data collection methods. A well-organized protocol is essential for ensuring consistency and reliability throughout the study. bioaccess® offers expertise in developing these protocols, ensuring compliance with country requirements and facilitating trial setup and approval processes. This encompasses feasibility and selection of research locations and lead investigators, which are essential for the success of the project.
Plan for Data Analysis: Establish a robust plan for data analysis, specifying the statistical methods and software to be utilized, such as SPSS or R. This plan should align with the project's objectives and ensure that the analysis can adequately address the research question. Incorporating statistical considerations, such as those highlighted in the external sources, will enhance the rigor of your research design. Furthermore, bioaccess® offers extensive project management and reporting services, which are crucial for monitoring project status and results efficiently.

Informed Consent: It is essential that all individuals provide informed consent, fully understanding the nature of the research, potential risks, and their right to withdraw at any moment. Recent research indicates that while 97.5% of individuals comprehend confidentiality, only 4.8% grasp the concept of placebo. This statistic underscores the necessity for clear communication throughout this process.
Ethics Review: Submit your research protocol to an Institutional Review Board (IRB) or Ethics Committee for thorough review and approval. This critical step ensures that the study adheres to ethical standards, safeguarding participant rights and welfare. Members of ethics committees emphasize that a robust ethics review process is vital for maintaining public trust in research involving patients.
Compliance with Regulations: Familiarize yourself with local and international regulations governing clinical trials, including the Declaration of Helsinki and Good Clinical Practice (GCP) guidelines. Adhering to these regulations is essential for ethical conduct and the integrity of the research.
Monitoring and Reporting: Establish a comprehensive plan for tracking the research's progress and reporting any adverse events or deviations from the protocol to the appropriate regulatory bodies. Effective monitoring not only ensures the safety of participants but also enhances the credibility of the research findings.

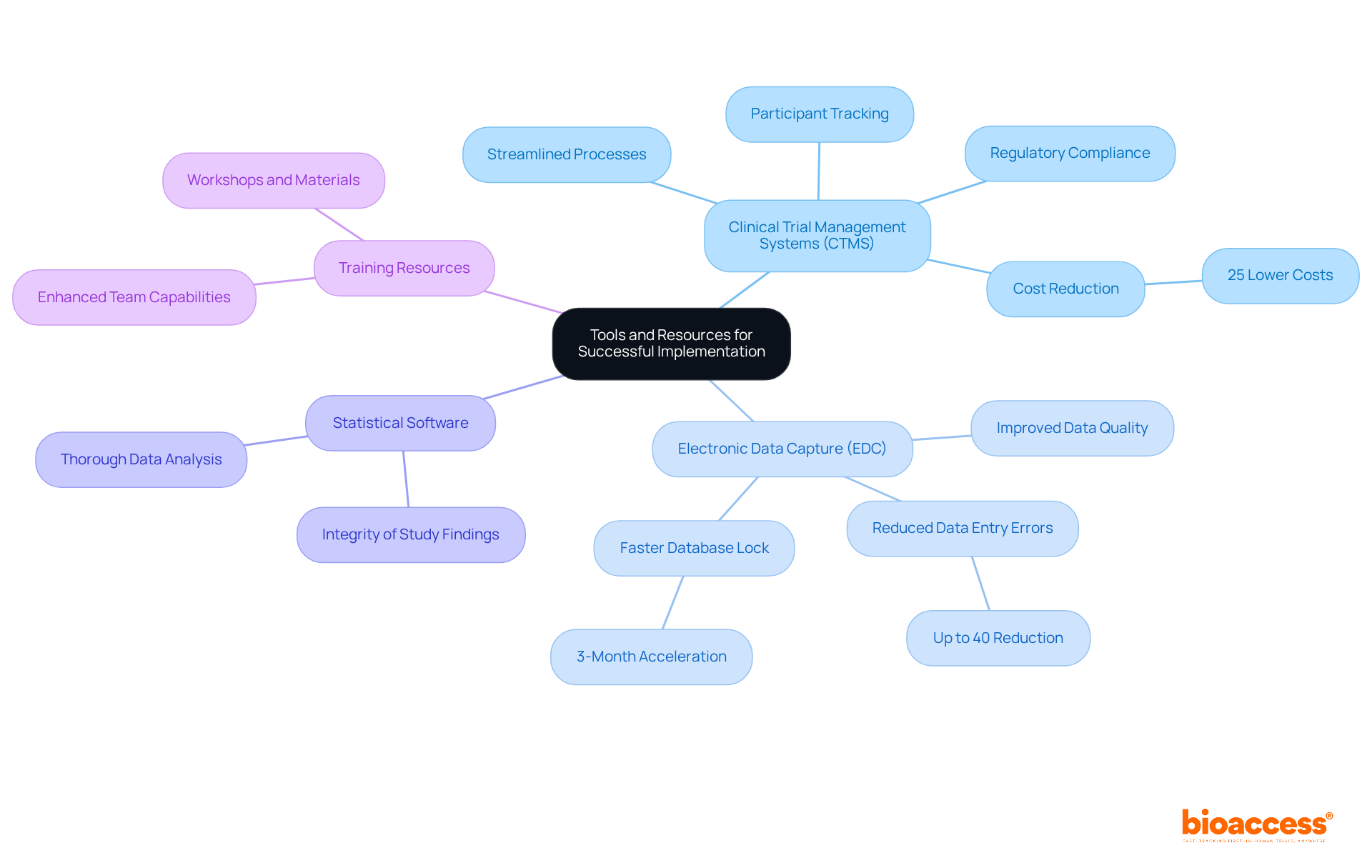
Clinical Trial Management Systems (CTMS): Leverage CTMS software to proficiently manage research logistics, encompassing participant tracking, data collection, and compliance documentation. These systems are essential for streamlining processes and ensuring adherence to regulatory requirements, thereby enhancing overall efficiency.
Electronic Data Capture (EDC): Adopt EDC systems to enable precise data entry and collection. EDC has become a preferred method in clinical trials, significantly reducing data entry errors by up to 40% and accelerating database lock times by 4%. This technology not only elevates data quality but also expedites the pace of testing execution.
Statistical Software: Employ robust statistical analysis software (e.g., SAS, R, SPSS) for thorough data analysis. This approach guarantees that necessary statistical tests can be performed to evaluate results effectively, thereby bolstering the integrity of study findings.
Training Resources: Utilize training materials and workshops focused on trial design and management. Continuous education enhances your team's capabilities and comprehension, equipping them to navigate the complexities of modern healthcare research.
Designing a successful placebo control study is a multifaceted endeavor that necessitates a nuanced understanding of research methodologies, ethical considerations, and effective implementation strategies. By mastering the essential components of this research design, researchers can yield reliable and impactful results that significantly contribute to the advancement of medical knowledge and treatment options.
Key elements discussed in this guide encompass:
Moreover, the paramount importance of informed consent, ethics review, and regulatory compliance cannot be overstated, as these elements ensure the integrity of the study and the protection of participant rights. The utilization of modern tools and resources, such as Clinical Trial Management Systems and Electronic Data Capture, further enhances the efficiency and accuracy of data collection and management.
Ultimately, the significance of placebo-controlled studies resides in their capacity to discern the true effects of treatments, paving the way for innovations in healthcare. Researchers are encouraged to embrace these methodologies and ethical practices, fostering a culture of rigor and transparency in clinical trials. By doing so, they not only enhance the credibility of their findings but also contribute to the broader goal of improving patient outcomes and advancing medical science.
What is a placebo-controlled study?
A placebo-controlled study is a research design where one group receives an active treatment while another group receives a placebo, an inactive substance that mimics the treatment. This method helps evaluate the efficacy of the treatment by comparing outcomes between the two groups.
Why is the placebo effect significant in clinical studies?
The placebo effect is significant because individuals may experience perceived improvements in their condition due to their expectations. Research shows that approximately 35% of patients may respond positively to placebo treatment, which can influence clinical outcomes.
What are the key methodologies used in placebo-controlled studies?
Key methodologies include randomization, which minimizes bias by allocating individuals to treatment groups randomly, and blinding, where individuals and/or researchers are unaware of group assignments to enhance the reliability of findings.
Can you provide an example of a placebo-controlled study?
A recent double-blind, placebo-controlled study assessed an Ayurvedic formulation for stress relief, finding significant reductions in anxiety levels in individuals receiving the active treatment compared to those on the placebo.
What ethical considerations are involved in placebo studies?
Ethical considerations include the need for informed consent, which must communicate the possibility of receiving a placebo. It becomes unethical to continue assigning participants to a placebo once there is evidence supporting the effectiveness of the experimental therapy, and placebos should not be used if it means withholding effective treatment for serious conditions.
How have placebo-controlled studies been applied in the Medtech sector?
Successful examples include the Novavax COVID-19 vaccine study, which utilized a robust placebo-controlled design that contributed to its authorization and recognition as a significant advancement in vaccination efforts.
What expertise does bioaccess® offer in clinical trials?
Bioaccess® has over 20 years of expertise in managing clinical trials, including Early-Feasibility, First-In-Human, and Post-Market Follow-Up Studies, helping clients navigate the complexities of clinical research confidently.