


The article delineates a structured approach to effectively address FDA Form 483 responses, a critical aspect for ensuring compliance and maintaining operational integrity. It underscores the significance of:
This process not only demonstrates a commitment to regulatory standards but also serves as a preventive measure against future violations.
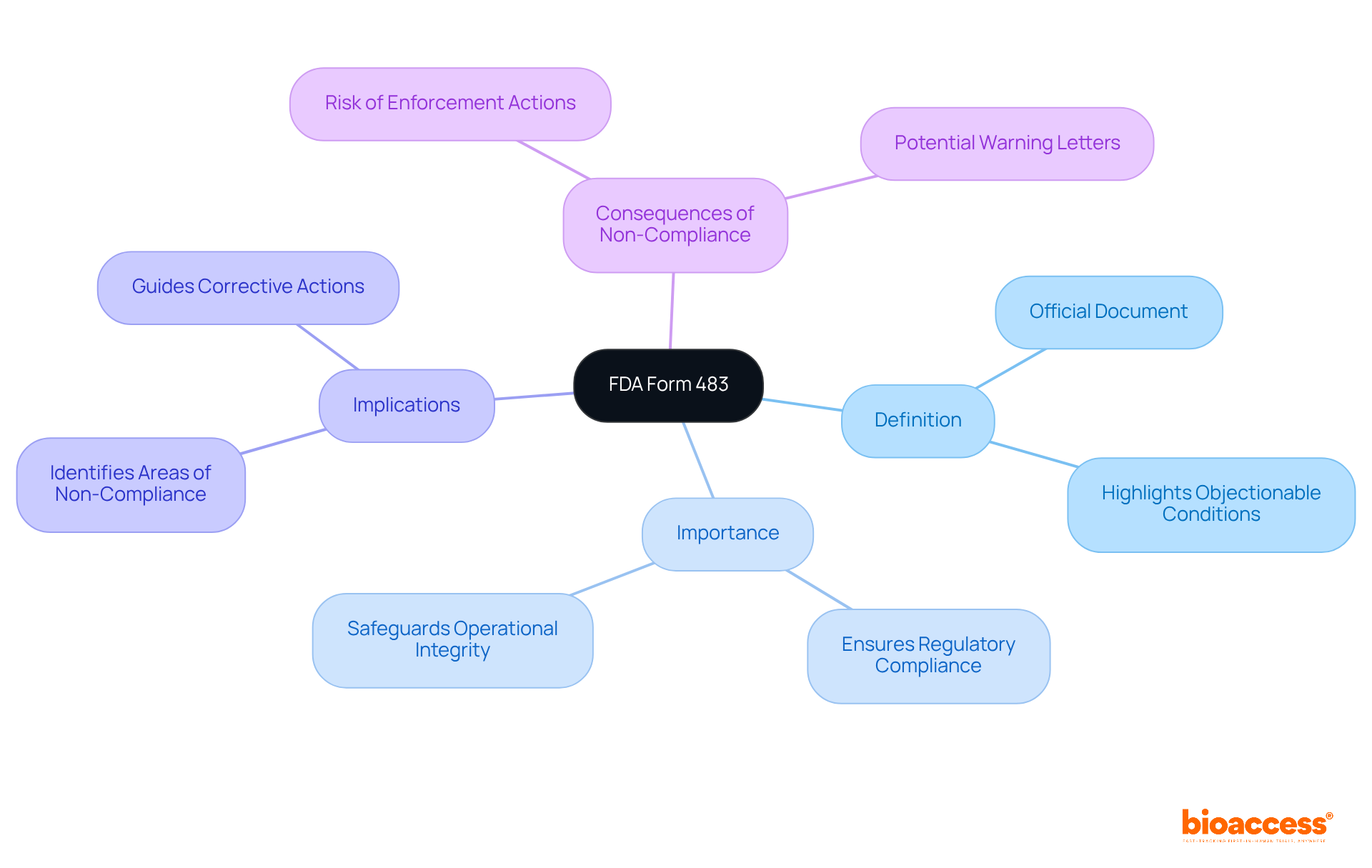
The FDA Form 483 serves as a critical touchpoint for companies navigating the complex landscape of regulatory compliance, highlighting areas of concern identified during inspections. Addressing this form effectively transcends mere compliance; it represents a significant opportunity for organizations to showcase their commitment to quality and patient safety. However, how can companies ensure their responses not only meet regulatory expectations but also bolster their operational integrity? This article outlines four essential steps to craft a robust response to FDA Form 483, empowering organizations to navigate potential pitfalls and enhance their compliance posture.
The FDA Form 483, officially referred to as the Notice of Inspectional Observations, is a critical document issued to a company's management at the conclusion of an FDA inspection. This form enumerates conditions or practices that FDA investigators find objectionable. Understanding the FDA Form 483 is essential, as it highlights areas where a company may not fully comply with regulatory standards.
Failure to adequately address the comments within can lead to severe repercussions, including warning letters or even enforcement actions. Therefore, grasping the significance of the FDA Form 483 is the first step toward ensuring compliance and safeguarding the integrity of your operations.
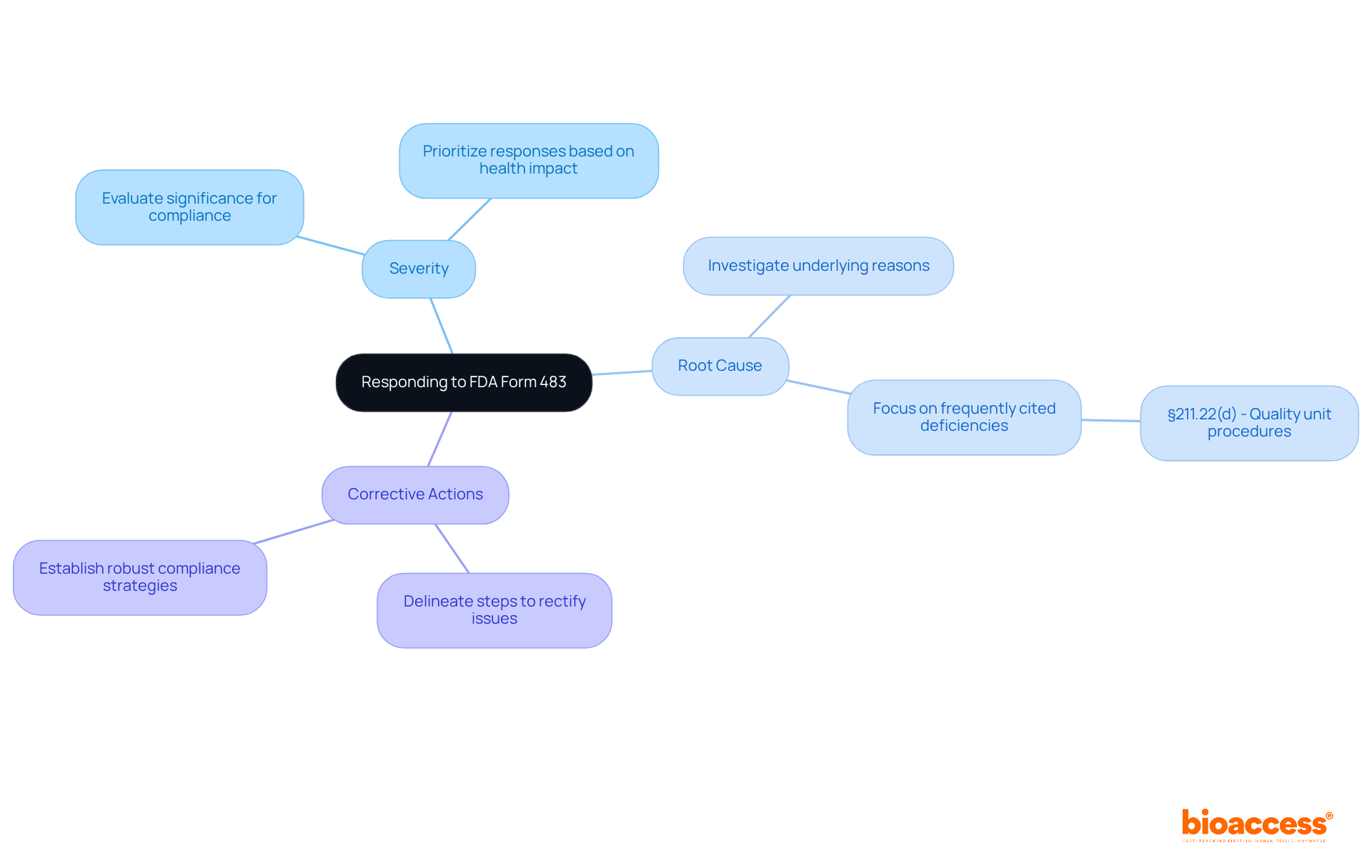
Begin by meticulously analyzing the FDA Form 483 and categorizing the findings into crucial areas such as regulatory failures, procedural lapses, and potential threats to patient safety. For each observation, consider the following key elements:
Severity: Evaluate the significance of the finding concerning compliance and patient safety. This evaluation aids in prioritizing responses based on the potential impact on health outcomes. As Barbara Unger noted, the frequency of FDA Form 483 issued to pharmaceutical companies has consistently risen, underscoring the importance of promptly addressing these remarks.
Root Cause: Investigate the underlying reasons for each finding. Grasping the root cause is vital for formulating effective corrective actions and preventing recurrence. In fiscal year 2019, the most frequently cited regulatory deficiency pertained to §211.22(d), emphasizing the necessity for documented procedures relevant to the quality unit.
Corrective Actions: Clearly delineate the steps to be taken to rectify each observation. This should encompass specific measures to address the identified issues and enhance compliance moving forward. The increase in FDA Form 483 issued, from 716 in 2018 to 779 in 2019, highlights the growing scrutiny from the FDA, making it crucial to establish a robust strategy for addressing concerns regarding FDA Form 483.
This structured approach not only ensures that your response is thorough but also demonstrates a commitment to resolving the identified issues and improving overall quality systems. By focusing on these components, organizations can effectively navigate the complexities of the FDA Form 483 feedback and bolster their compliance stance, aligning with the fundamental elements of a robust Pharmaceutical Quality System as emphasized by the FDA.
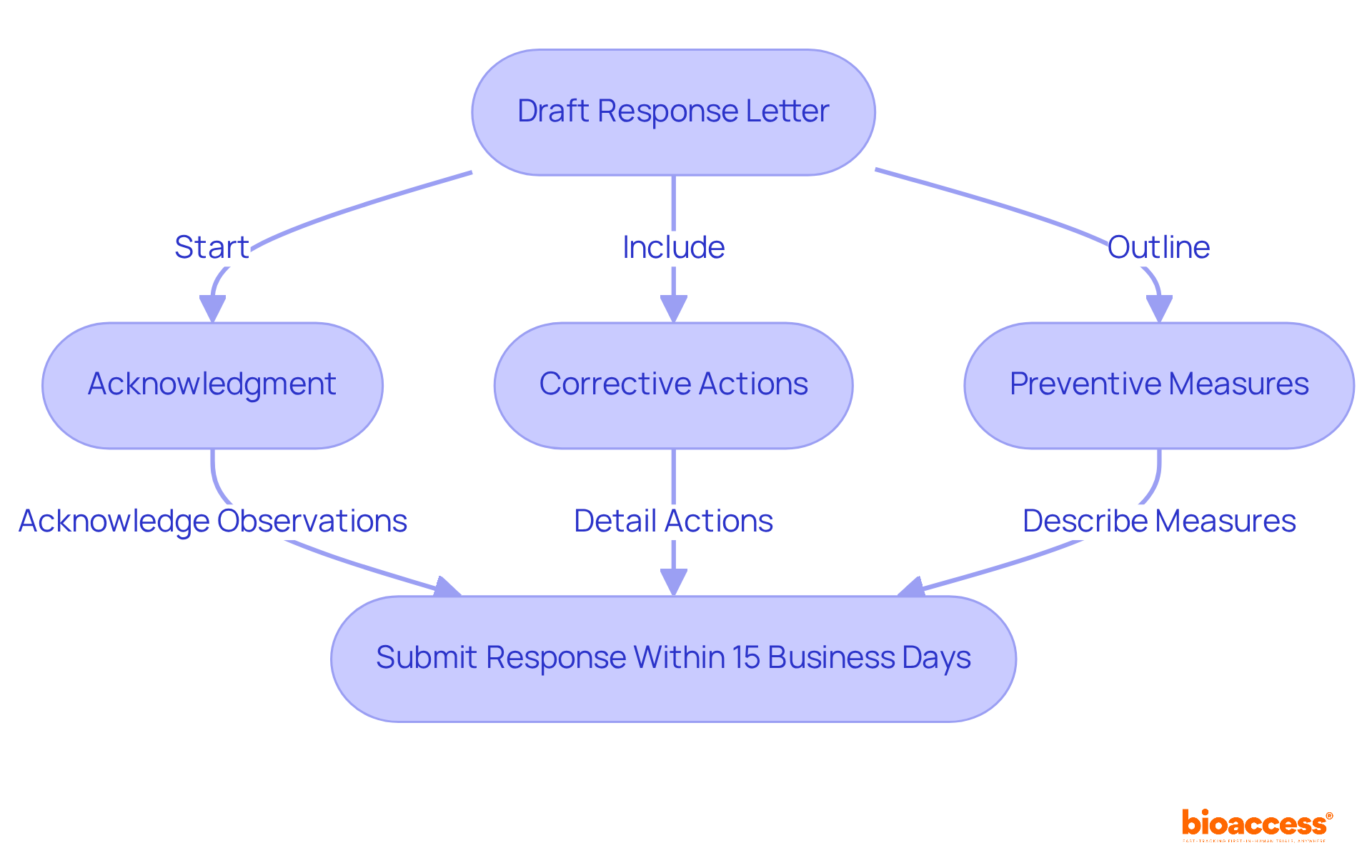
When drafting your response letter, it is essential to ensure a structured and professional format. Begin with a concise introduction that acknowledges receipt of the FDA Form 483. Address each remark individually, incorporating the following elements:
Additionally, ensure that your response is submitted within 15 business days to avoid a Warning Letter, as timely submission demonstrates procedural discipline and respect for regulatory timelines. Utilize clear and concise language, maintaining a respectful tone throughout the letter. This approach not only showcases your commitment to addressing the FDA's concerns but also strengthens your organization's dedication to regulatory adherence and the management of FDA Form 483. As Vivek Gera mentions, replying to the FDA Form 483 letters is a strategic opportunity to reinforce your organization's dedication to quality, integrity, and regulatory standards.
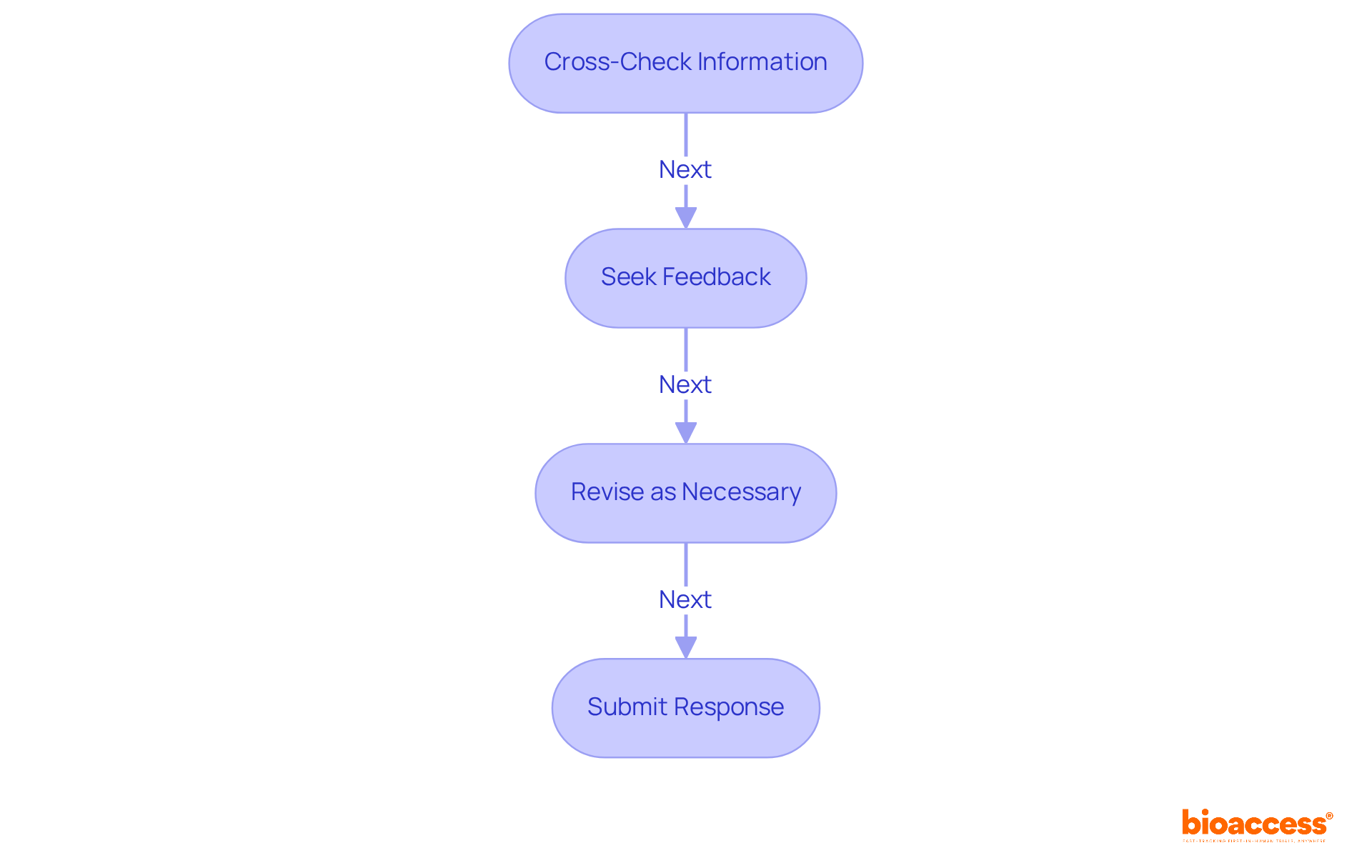
Before submitting your reply regarding the observations in FDA Form 483, it is essential to conduct a meticulous review to ensure both accuracy and completeness. Consider the following steps:
Cross-Check Information: Rigorously verify that all facts, figures, and corrective actions outlined in your reply are precise and reflect the current state of adherence. The FDA expects manufacturers to demonstrate closed-loop traceability of all quality system processes, underscoring the importance of precision in your reply.
Seek Feedback: Engage colleagues or compliance experts to review your reply. Their insights can enhance clarity and ensure that all necessary details are included. Research indicates that organizations implementing structured review processes often see a marked decrease in the likelihood of receiving further observations or warnings.
Revise as Necessary: Incorporate any feedback received, making adjustments to enhance the reply while maintaining a professional tone. A well-reviewed reply not only demonstrates your commitment to compliance but also strengthens your credibility with the FDA. Insufficient replies can lead to escalated actions, including warning letters, so it is essential to ensure thoroughness.
Additionally, remember that you must respond to FDA 483 observations within 15 business days. Supplying copies of documents and/or records as part of your reply is also a crucial element of compliance. By prioritizing accuracy and thoroughness in your response, you position your organization favorably in the eyes of regulatory authorities.
Addressing an FDA Form 483 is a critical process that underscores a company's commitment to regulatory compliance and operational integrity. Understanding the implications of this form and following a structured approach enables organizations to effectively navigate the complexities involved in responding to FDA observations. A well-prepared response not only mitigates potential risks but also fosters a culture of continuous improvement and adherence to quality standards.
This article outlines key steps in this process, including:
Each step is designed to ensure that corrective actions are not only taken but are also sustainable, thereby preventing future compliance issues. Emphasizing the significance of timely and accurate responses reinforces the need for vigilance in maintaining regulatory standards.
Ultimately, the ability to address FDA Form 483 observations effectively can determine the trajectory of a company’s compliance journey. It serves as an opportunity for organizations to showcase their dedication to quality and regulatory standards, transforming potential setbacks into a pathway for improvement. Engaging with this process proactively can significantly enhance an organization’s reputation and operational resilience in the competitive landscape of regulated industries.
What is the FDA Form 483?
The FDA Form 483, officially known as the Notice of Inspectional Observations, is a document issued to a company's management at the end of an FDA inspection, detailing conditions or practices that FDA investigators find objectionable.
Why is understanding the FDA Form 483 important?
Understanding the FDA Form 483 is important because it highlights areas where a company may not fully comply with regulatory standards, which is essential for ensuring compliance and maintaining the integrity of operations.
What can happen if a company fails to address the comments in the FDA Form 483?
If a company fails to adequately address the comments in the FDA Form 483, it can lead to severe repercussions, including warning letters or even enforcement actions from the FDA.