


The article delineates four crucial steps for successfully navigating the New Drug Application (NDA) submission process:
Each step underscores the significance of meticulous data collection, strict adherence to regulatory requirements, and effective communication with the FDA to bolster the likelihood of approval. This guidance is substantiated by real-world examples and statistical success rates, reinforcing the importance of these practices in the clinical research landscape.
Navigating the New Drug Application (NDA) submission process represents a pivotal milestone for any pharmaceutical company aspiring to introduce innovative therapies to the market. With a noteworthy success rate of 86.1% for New Molecular Entities, a comprehensive understanding of the NDA process can significantly bolster the likelihood of approval.
However, this journey is laden with complexities—ranging from meticulous data collection to stringent regulatory compliance and effective communication with the FDA.
How can stakeholders ensure they are thoroughly prepared to confront these challenges and optimize their NDA submissions for success?
The New Drug Application (NDA) procedure serves as a formal request to the FDA for approval to market a new medication in the United States, representing a pivotal milestone in the drug development lifecycle. This procedure encapsulates years of rigorous research and clinical trials, making it essential for stakeholders to grasp its intricacies to ensure that all necessary data and documentation are meticulously prepared and submitted.
Key components of the NDA encompass:
Each element plays a vital role in the FDA's assessment of the drug's safety and efficacy. Recent statistics reveal that the probability of success for New Molecular Entities (NME) from NDA to approval is approximately 86.1%, underscoring the necessity of a well-structured application. Moreover, the NDA must convincingly demonstrate that the drug is safe and effective for its intended use, which is why a comprehensive understanding of the procedure is critical for success.
Real-world examples further illustrate the NDA's significance; for instance, drugs that utilize biomarkers generally exhibit higher success rates throughout development phases compared to those that do not. This highlights the importance of strategic planning in the NDA filing process. Additionally, FDA representatives emphasize that a thorough NDA submission is crucial not only for evaluation but also for enhancing the likelihood of approval, making it a vital step in introducing innovative therapies to the market.
At bioaccess®, we specialize in advancing medical devices through our extensive experience in clinical trials, including:
Each of these study types plays a crucial role in generating the robust clinical data necessary for a successful NDA submission. By leveraging our expertise, stakeholders can navigate the complexities of the NDA filing process more efficiently, ultimately enhancing their prospects of obtaining regulatory approval.

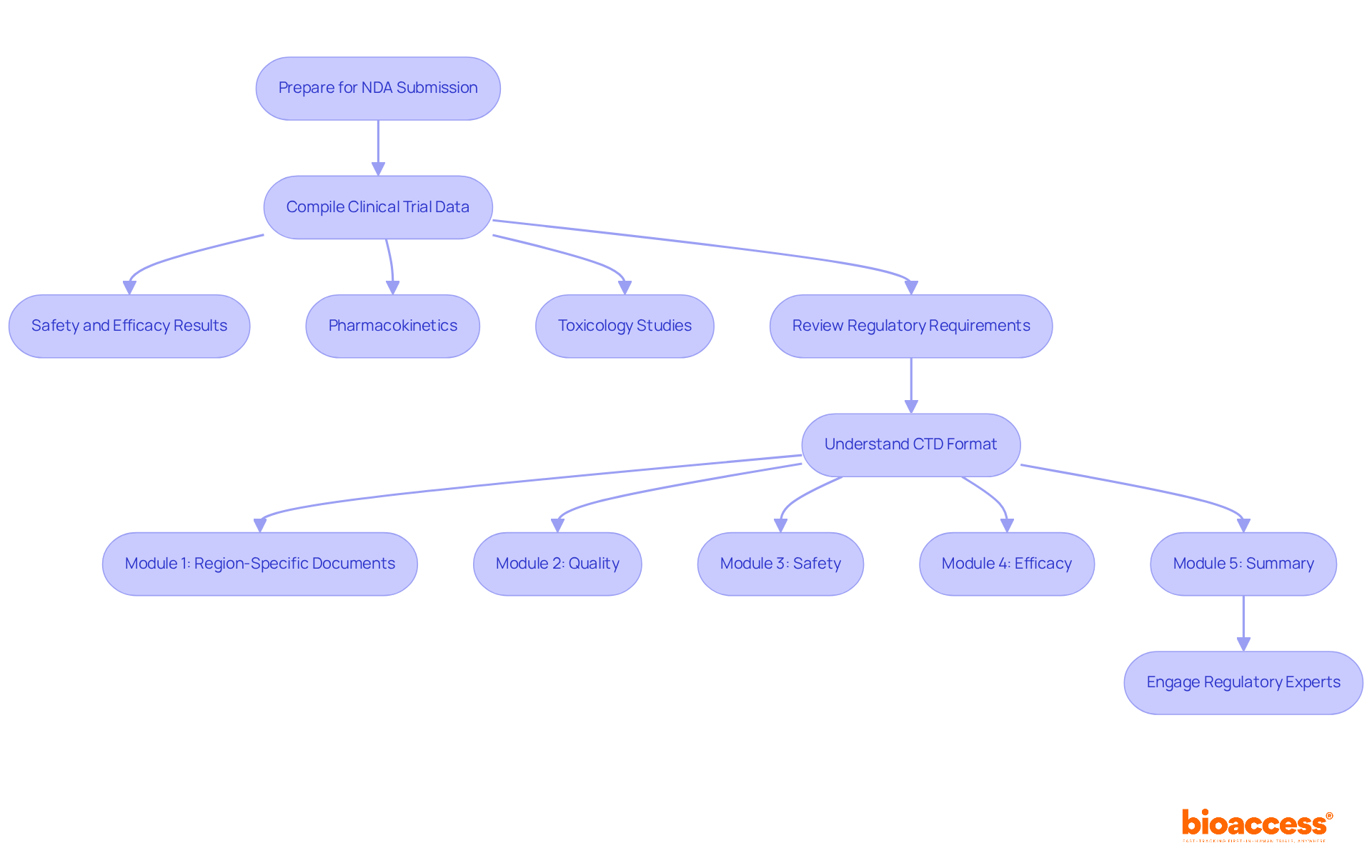
Preparing for an NDA submission requires meticulous attention to data collection and strict adherence to regulatory requirements. Begin by compiling all relevant clinical trial data, including:
Clearly arranging this information is essential, as it simplifies the review phase and enables a comprehensive assessment by regulatory authorities.
Familiarize yourself with the FDA's regulatory requirements for NDA submission, which include essential documentation such as the Common Technical Document (CTD) format. The CTD is organized into five modules, outlining the essential elements for a successful application. Engaging with regulatory experts or consultants can provide valuable insights and ensure compliance with all guidelines. By preparing thoroughly and grasping the intricacies of the NDA submission, you can significantly improve the chances of a successful entry.
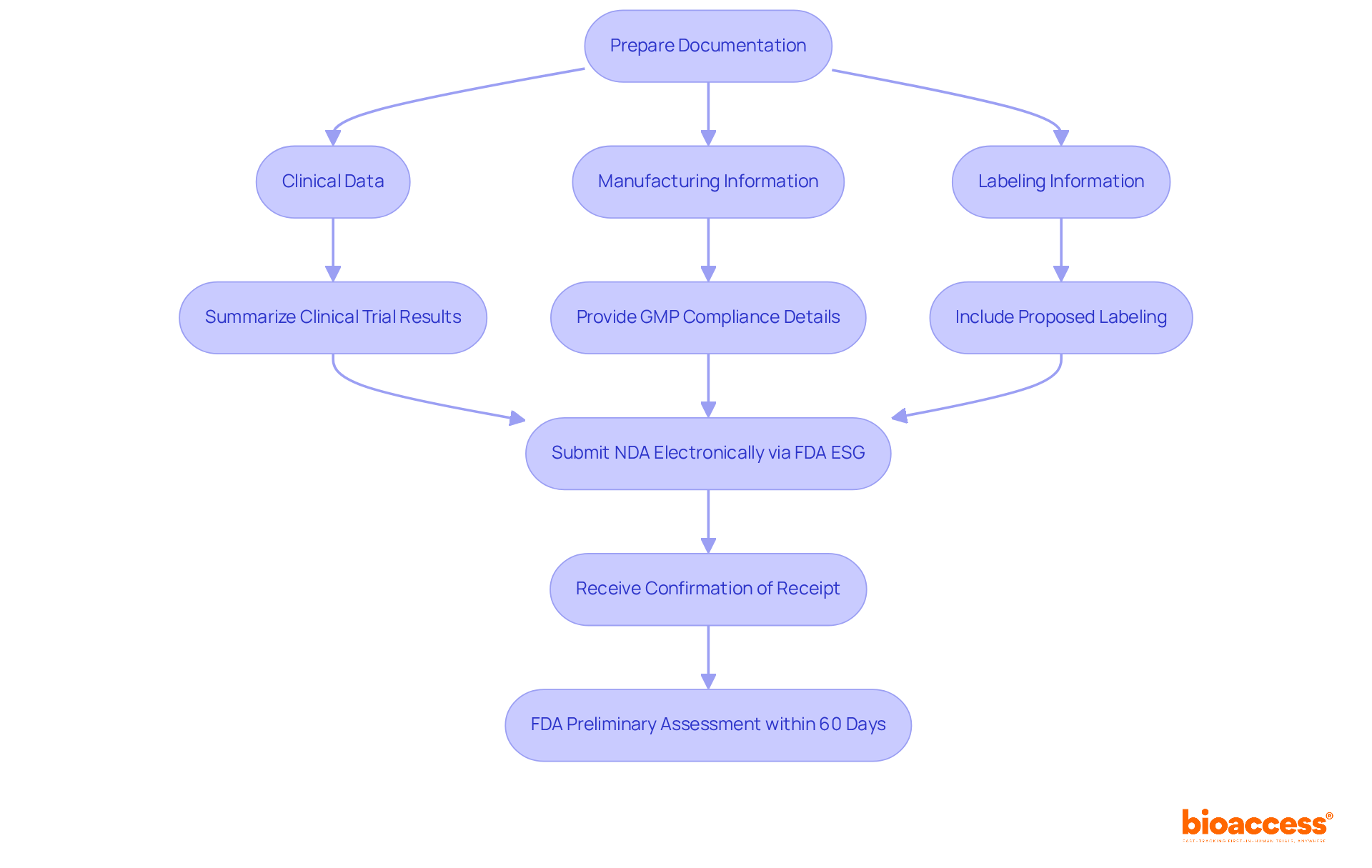
Once all necessary documentation is prepared, executing the NDA filing is the next critical step. Begin by ensuring that all key components are meticulously included:
After compiling these components, ensure the NDA submission is done electronically through the FDA's Electronic Submission Gateway (ESG). This platform is intended to simplify the workflow for entries, and using it efficiently can greatly decrease mistakes in submissions. Ensure that you receive confirmation of receipt from the FDA, as this serves as proof of your NDA submission. After the filing, the FDA will perform a preliminary assessment for thoroughness within 60 days, which is a crucial milestone in the NDA submission process.
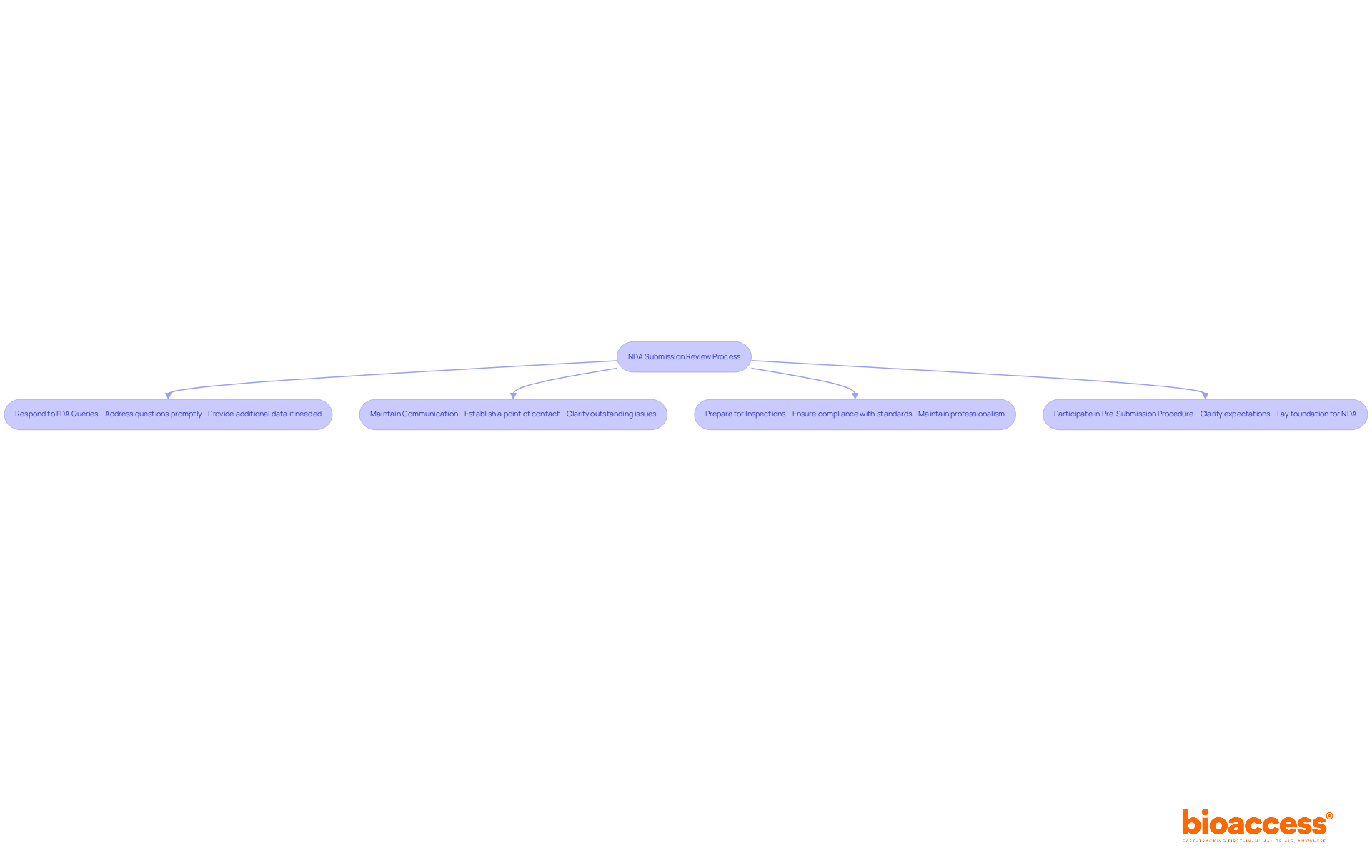
Following the nda submission of your New Drug Application, the FDA initiates a review process that typically spans around 10 months. This critical phase necessitates proactive management of your entry. Focus on these key responsibilities:
Respond to FDA Queries: Be ready to promptly address any questions or requests for additional information from the FDA. This may involve providing further data or clarifications regarding your submission. Statistics reveal that timely responses can significantly influence the review timeline, as companies have 180 days to react to an AI (Additional Information) letter from the FDA.
Maintain Communication: Establish a specific point of contact with the FDA to facilitate smooth communication throughout the review phase. Effective communication can clarify outstanding issues and enhance the likelihood of a favorable outcome.
Prepare for Inspections: The FDA may conduct facility examinations as part of the evaluation process. Ensure that all manufacturing practices adhere to regulatory standards. Past experiences underscore the importance of professionalism during inspections; confrontational behavior can exacerbate situations negatively.
Participate in Pre-Submission Procedure: Engaging in the pre-submission procedure can significantly elevate the chances of approval. This proactive measure allows for clarification of expectations and requirements, laying a solid foundation for your NDA submission.
In addition to these responsibilities, consider the comprehensive clinical trial management services offered by bioaccess, which include feasibility studies, site selection, compliance reviews, trial setup, import permits, project management, and reporting. Utilizing these services can streamline your clinical trial procedures and ensure adherence to regulatory requirements, ultimately enhancing the likelihood of a successful NDA submission. By effectively managing these post-submission responsibilities alongside robust clinical trial management, you can navigate the review process more efficiently.
Navigating the New Drug Application (NDA) submission process is a pivotal endeavor for stakeholders aiming to introduce new medications to the market. Understanding the significance of this process—ranging from meticulous data collection to executing a well-structured submission—is essential for achieving regulatory approval. The NDA not only represents the culmination of extensive research and clinical trials but also serves as a gateway for innovative therapies to reach patients in need.
Key insights discussed throughout the article highlight the importance of thorough preparation, including:
Each step, from compiling clinical trial results to managing post-submission responsibilities, plays a crucial role in enhancing the likelihood of approval. Engaging with regulatory experts and utilizing comprehensive clinical trial management services can further streamline the NDA submission process, ensuring that all necessary components are meticulously addressed.
Ultimately, the NDA submission process is not merely a bureaucratic hurdle; it is a critical pathway that determines the future of new therapies. By prioritizing strategic planning, thorough preparation, and proactive management of the review phase, stakeholders can significantly increase their chances of success. Embracing these practices will not only facilitate the approval of new drugs but also contribute to the advancement of healthcare and the well-being of patients who rely on these innovative treatments.
What is the New Drug Application (NDA) process?
The NDA process is a formal request to the FDA for approval to market a new medication in the United States, representing a critical milestone in the drug development lifecycle.
What are the key components of an NDA?
The key components of an NDA include clinical data, manufacturing information, and labeling details, all of which are essential for the FDA's assessment of the drug's safety and efficacy.
What is the success rate for New Molecular Entities (NME) from NDA to approval?
The probability of success for New Molecular Entities (NME) from NDA to approval is approximately 86.1%.
Why is a comprehensive understanding of the NDA procedure important?
A comprehensive understanding of the NDA procedure is critical for success because it ensures that all necessary data and documentation are meticulously prepared and submitted, demonstrating that the drug is safe and effective for its intended use.
How do biomarkers influence the success rate of drugs in the NDA process?
Drugs that utilize biomarkers generally exhibit higher success rates throughout development phases compared to those that do not, highlighting the importance of strategic planning in the NDA filing process.
What role does a thorough NDA submission play in the approval process?
A thorough NDA submission is crucial not only for evaluation by the FDA but also for enhancing the likelihood of approval, making it a vital step in introducing innovative therapies to the market.
What types of studies does bioaccess® specialize in to support NDA submissions?
Bioaccess® specializes in various types of studies, including Early-Feasibility Studies (EFS), First-In-Human Studies (FIH), Pilot Studies, Pivotal Studies, and Post-Market Clinical Follow-Up Studies (PMCF), all of which are important for generating robust clinical data necessary for a successful NDA submission.