


The article delineates the essential steps for acquiring regulatory dossier services in Mexico, underscoring the critical nature of comprehending regulatory requirements and selecting a qualified service provider. It elaborates on the necessary components of a regulatory dossier, the classification of medical products by COFEPRIS, and the criteria for choosing a service provider. Each of these elements is vital for ensuring compliance and facilitating a successful approval process.
Navigating the complex landscape of medical product regulations in Mexico is a formidable challenge, particularly for those unfamiliar with the Federal Commission for the Protection against Sanitary Risks (COFEPRIS) requirements. Grasping the critical components of a regulatory dossier is vital for ensuring compliance and facilitating a more efficient approval process. Given the evolving guidelines and the necessity for meticulous preparation, how can one effectively procure regulatory dossier services in Mexico while avoiding common pitfalls? This guide delineates the essential steps to streamline the purchasing process and significantly enhance the likelihood of successful submissions.
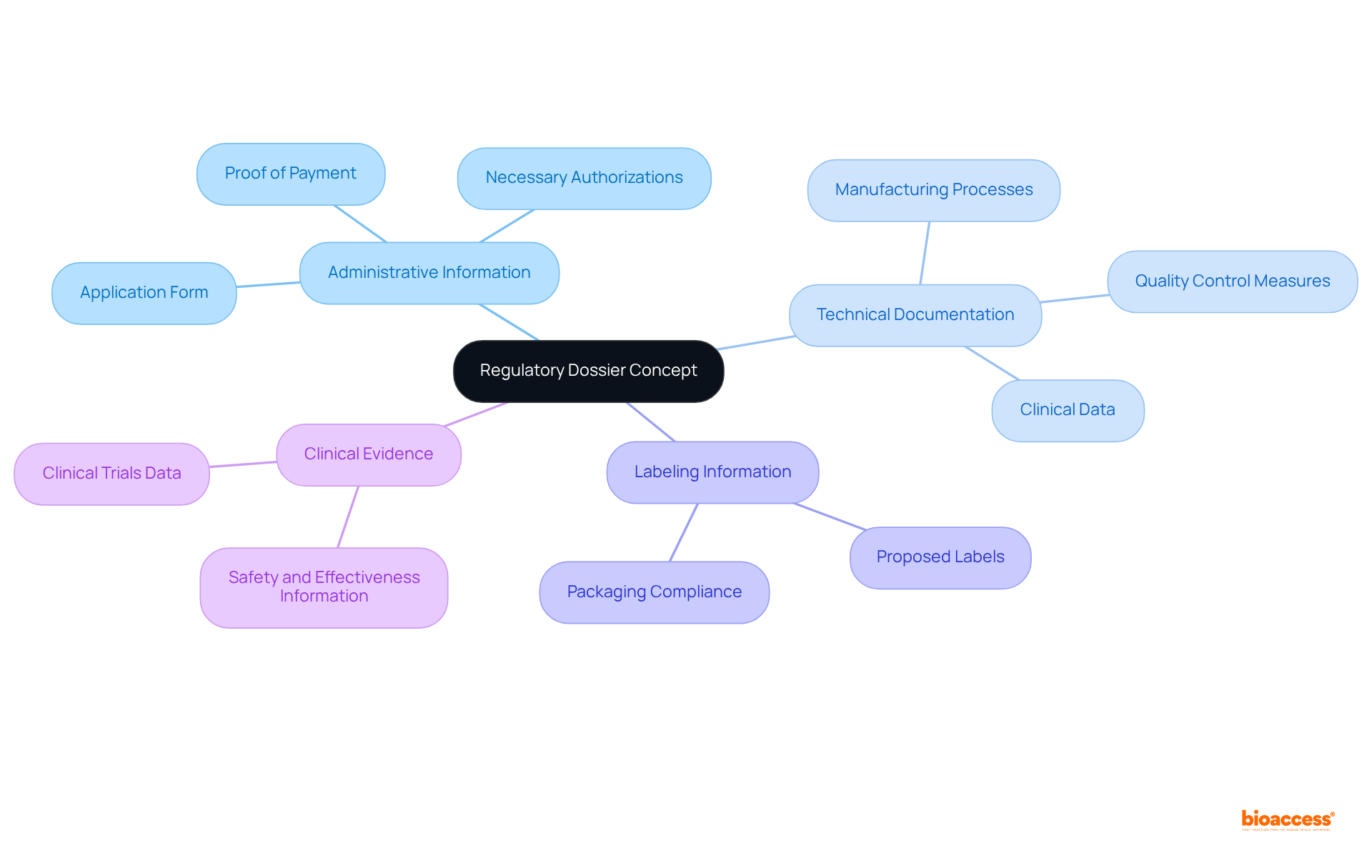
A compliance file is a vital collection of documents that outlines essential information regarding a medical product, including its safety, effectiveness, and quality. In Mexico, to successfully obtain approval, it is crucial to purchase regulatory dossier service Mexico and comply with the Federal Commission for the Protection against Sanitary Risks (COFEPRIS). The key components of a regulatory dossier typically include:
Understanding these elements is critical for ensuring that your purchase regulatory dossier service Mexico complies with standards, thereby facilitating a smoother approval process. As the regulatory landscape continues to evolve, particularly with anticipated updates in 2025, it is imperative to remain informed about COFEPRIS guidelines to effectively purchase regulatory dossier service Mexico. Successful submissions often hinge on a comprehensive understanding of these document components, prompting many to purchase regulatory dossier service Mexico, as evidenced by case studies that highlight the importance of meticulous preparation. Regulatory experts stress that it is vital to purchase regulatory dossier service Mexico to ensure a compliant and effective Quality Management System (QMS), which is essential for maintaining the quality and safety of medical products, underscoring the necessity for diligence in dossier preparation.
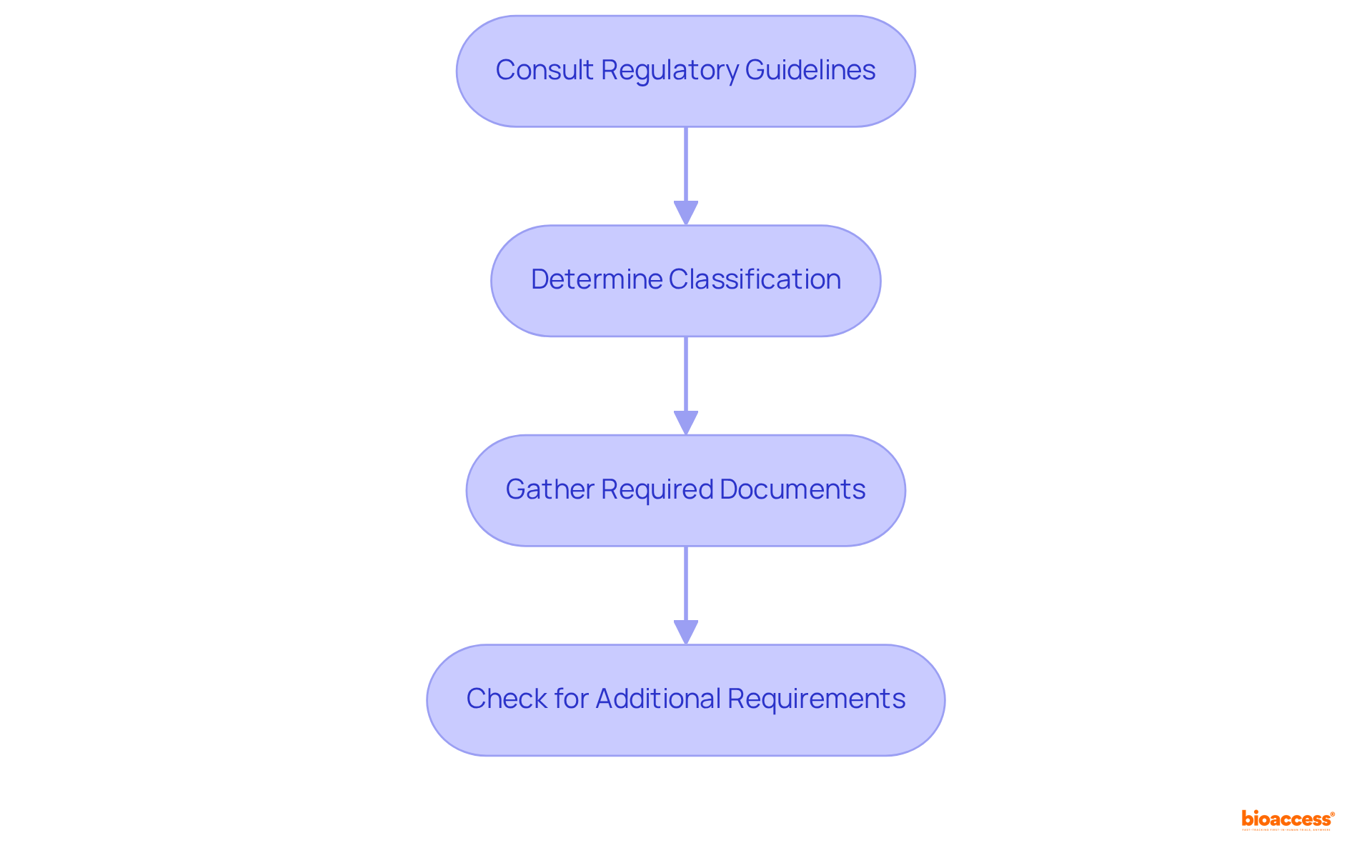
To effectively navigate the compliance framework in Mexico, it is crucial to understand the specific demands outlined by the health authority. The following steps are essential:
Consult Regulatory Guidelines: Begin by reviewing the official guidelines provided by the health authority specific to your product category, such as medical devices or pharmaceuticals. Notably, recent updates as of July 2025 have refined the classification criteria for equipment registration, making it imperative to grasp these changes.
Determine Classification: It is essential to comprehend how your product is classified, as this will dictate the documentation and testing required. COFEPRIS categorizes medical devices into four risk-based classes: Class I, Class II, Class III, and Class IV. Class II devices, in particular, necessitate a more rigorous oversight process due to their moderate risk profile.
Gather Required Documents: Compile all necessary documentation, including:
Check for Additional Requirements: Be aware that some products may require further approvals or certifications, such as environmental assessments or local market studies. Engaging local experts or regulatory consultants can significantly enhance the quality of your submissions and aid in effectively navigating the regulatory landscape.
Understanding these requirements is vital; common documentation errors can lead to substantial delays in the approval process. For instance, 32% of FDA 510(k) submissions faced requests for additional information in 2022, underscoring the importance of meticulous preparation. By ensuring your file is comprehensive and compliant, you can facilitate a smoother path to market entry. Additionally, note that the average review duration for applications typically ranges from 3 to 8 months, but utilizing an Authorized Third Party can expedite this process to as little as 1 to 3 months.
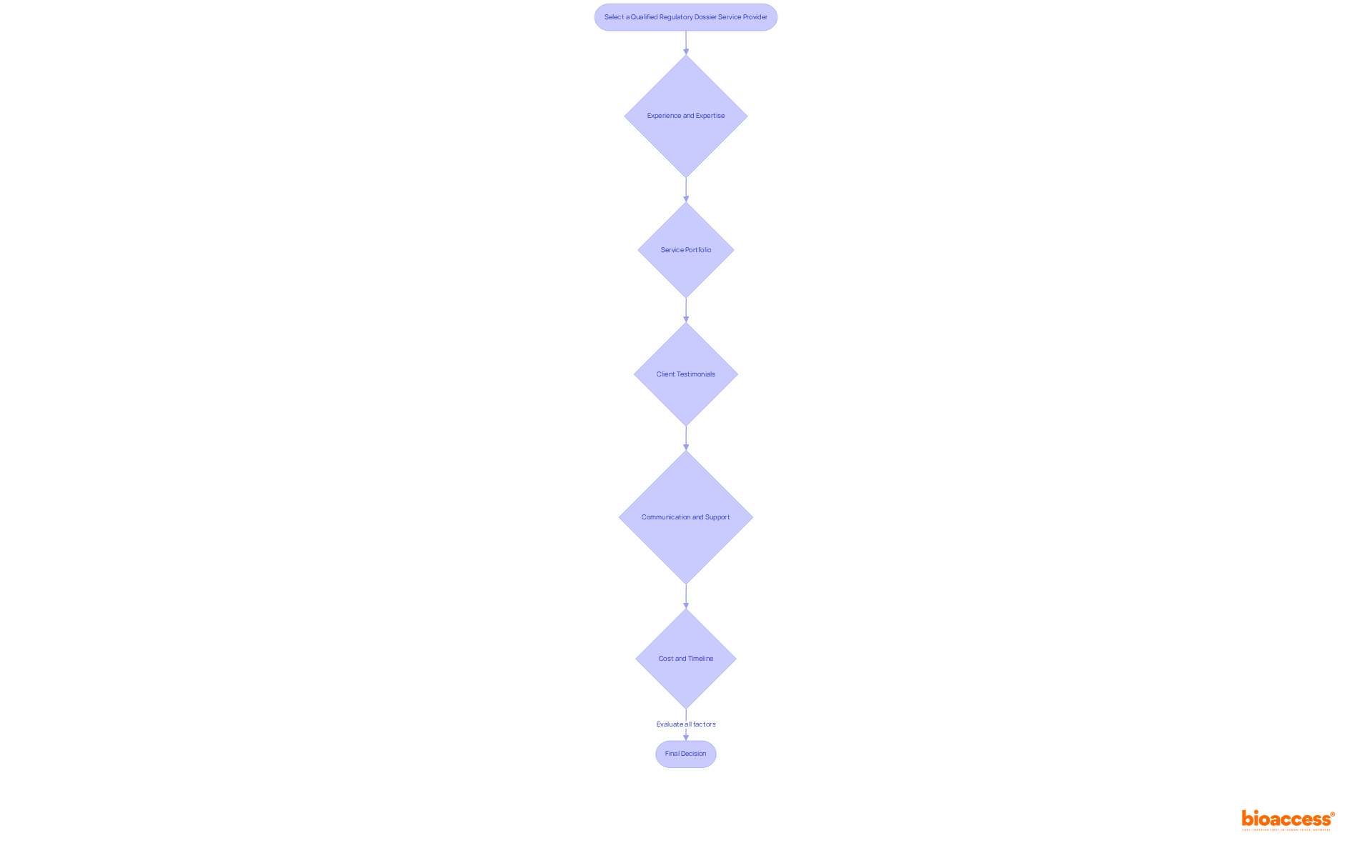
Selecting a qualified service provider to purchase regulatory dossier service Mexico for COFEPRIS submissions necessitates careful consideration of several essential factors.
Experience and expertise: Prioritize providers who can offer a purchase regulatory dossier service Mexico and have a solid track record in preparing regulatory documents specifically for the Mexican market. Their familiarity with COFEPRIS submissions is crucial, as it directly impacts the likelihood of successful approvals. As Maria I. Guaia emphasizes, "You should customize your portfolio to the Mexican requirements; even if all the necessary information is included, you shouldn’t just submit a portfolio that has been created for another market."
Service Portfolio: Ensure the supplier offers a comprehensive range of services, including dossier preparation, submission management, and post-submission support. This holistic approach can streamline the entire process of the purchase regulatory dossier service Mexico.
Client Testimonials: Investigate feedback from previous customers to assess the service's reliability and effectiveness. Positive reviews can suggest a supplier's ability to handle the intricacies of the regulatory body. Involving local specialists and regulatory advisors can also enhance dossier quality and simplify communications with the agency.
Communication and Support: Choose a service that emphasizes clear communication and ongoing assistance throughout the submission process. Given the complexities involved in regulatory submissions, this ensures you remain informed at every stage, which is vital for timely decision-making.
Cost and Timeline: Discuss pricing structures and expected timelines upfront. Grasping the typical duration required by compliance document service firms for submissions, which can differ greatly, will assist in aligning their services with your project requirements. For instance, COFEPRIS registration fees for New Molecules are approximately MXN 146,642, while Class III Medical Devices are around MXN 25,000.
By carefully assessing these elements, you can choose a compliance file service supplier that efficiently satisfies your compliance needs and enhances your likelihood of success when you purchase regulatory dossier service Mexico.
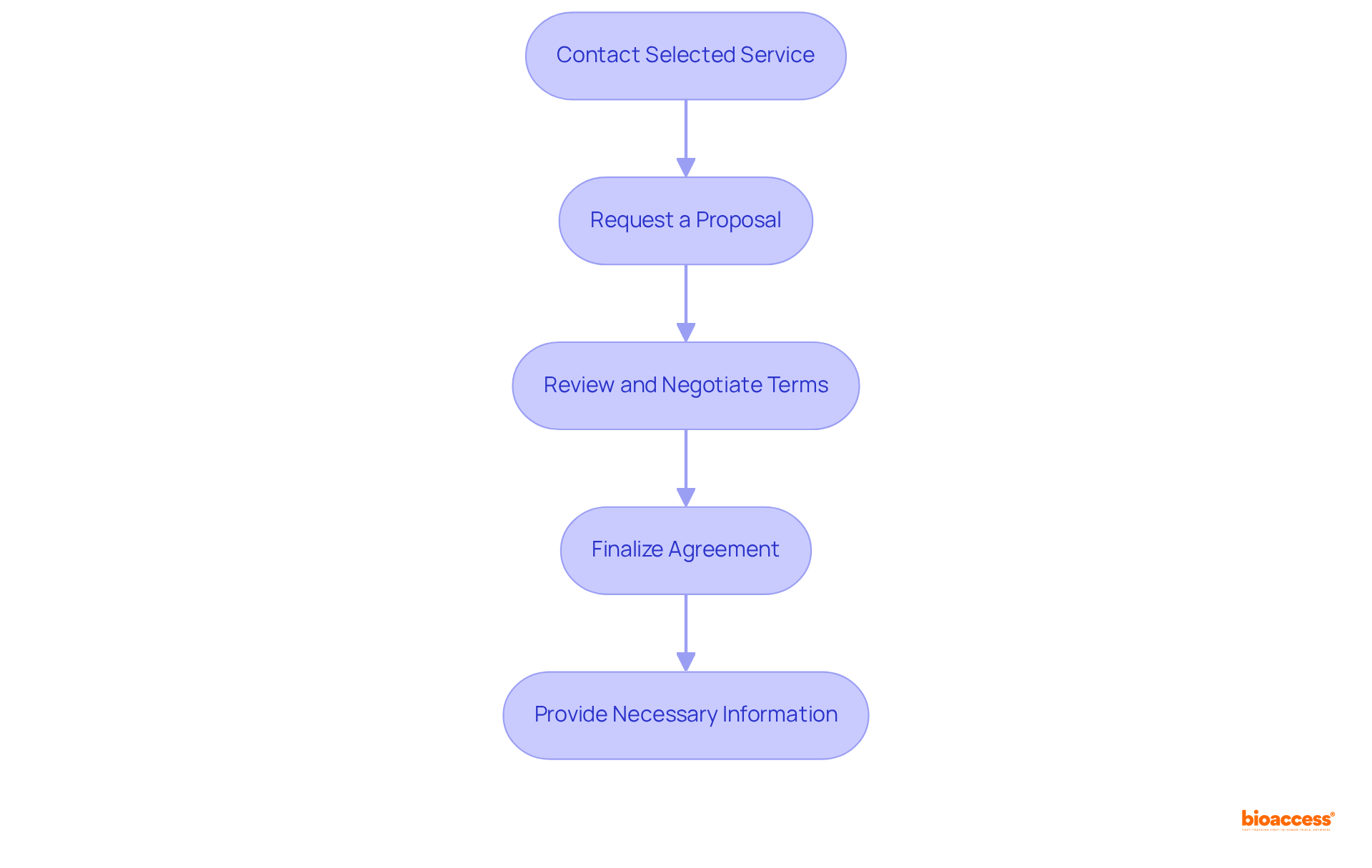
To initiate the purchase process for a regulatory dossier service in Mexico, follow these steps:
Contact Selected Service: Reach out to your chosen service entity to discuss your specific needs and confirm their capability to assist with your dossier preparation. Given that bioaccess® delivers ethical approvals in 4-6 weeks, it's crucial to ensure the supplier can meet your deadlines.
Request a Proposal: Ask for a detailed proposal that outlines the services offered, timelines, and costs involved. This proposal should demonstrate the supplier's comprehension of the compliance landscape, which is crucial for successful navigation through Mexico's approval processes.
Review and Negotiate Terms: Carefully review the proposal and negotiate any terms as necessary to ensure they align with your expectations. As noted by Ana Criado, Director of Regulatory Affairs, clear communication during this phase is vital to establish a mutually beneficial agreement.
Finalize Agreement: Once satisfied with the terms, finalize the agreement by signing a contract that outlines the scope of work, payment terms, and timelines. This contract serves as a foundation for your collaboration, ensuring both parties are aligned on expectations.
Provide Necessary Information: Supply the supplier with all required details and documentation to initiate the dossier preparation process. Prompt delivery of precise information is essential, as it can greatly influence the effectiveness of the submission process.
By following these steps, you can effectively initiate the purchase regulatory dossier service Mexico process and prepare for a successful submission, using the expertise of your selected provider to navigate the complexities of the Mexican market. Additionally, keep in mind that new GMP guidelines set to take effect on November 30, 2025, are expected to reduce approval times by as much as 30%, further enhancing the efficiency of your regulatory dossier service.
Purchasing a regulatory dossier service in Mexico is a multifaceted process that demands meticulous attention to detail and strict adherence to established guidelines. Understanding the components of the regulatory dossier, the specific requirements set by COFEPRIS, and the importance of selecting a qualified service provider are paramount to navigating these complexities effectively.
Familiarizing oneself with the regulatory landscape is essential, as is the need for thorough documentation. Engaging experienced service providers can significantly enhance the likelihood of achieving timely approvals for medical products. By adopting a structured approach—from grasping the regulatory dossier concept to initiating the purchase process—individuals and organizations can position themselves for success.
As the regulatory landscape in Mexico continues to evolve, staying informed about changes is critical. Collaborating with knowledgeable professionals and adhering to meticulous preparation can streamline the approval process. For those seeking to enter the Mexican market, taking proactive steps now will not only facilitate compliance but also strategically position products for successful entry and acceptance in a competitive environment.
What is a regulatory dossier?
A regulatory dossier is a collection of documents that outlines essential information regarding a medical product, including its safety, effectiveness, and quality.
Why is it important to purchase regulatory dossier service in Mexico?
Purchasing regulatory dossier service in Mexico is crucial for complying with the Federal Commission for the Protection against Sanitary Risks (COFEPRIS) and successfully obtaining approval for medical products.
What are the key components of a regulatory dossier?
The key components of a regulatory dossier include Administrative Information, Technical Documentation, Labeling Information, and Clinical Evidence.
What does the Administrative Information section include?
The Administrative Information section includes the application form, proof of payment, and any necessary authorizations required by COFEPRIS.
What is covered in the Technical Documentation?
The Technical Documentation provides detailed descriptions of the product, including manufacturing processes, quality control measures, and clinical data that support the product's claims.
What regulations must labeling information comply with in Mexico?
Labeling information must comply with Mexican regulations, ensuring clarity and adherence to established guidelines.
Why is Clinical Evidence important in a regulatory dossier?
Clinical Evidence is important as it contains information from clinical trials that demonstrate the product's safety and effectiveness, which is essential for regulatory assessment.
How can staying informed about COFEPRIS guidelines benefit regulatory submissions?
Staying informed about COFEPRIS guidelines can facilitate a smoother approval process and help ensure compliance with evolving regulatory standards, especially with anticipated updates in 2025.
What role does a Quality Management System (QMS) play in the regulatory dossier process?
A Quality Management System (QMS) is essential for maintaining the quality and safety of medical products, and purchasing regulatory dossier service in Mexico helps ensure a compliant and effective QMS.
What do regulatory experts emphasize regarding the preparation of regulatory dossiers?
Regulatory experts emphasize the importance of meticulous preparation of regulatory dossiers to ensure compliance and effectiveness in the approval process.