


The article outlines four essential steps for purchasing technical file translation into Brazilian Portuguese, emphasizing the importance of:
Each step is supported by specific strategies and insights:
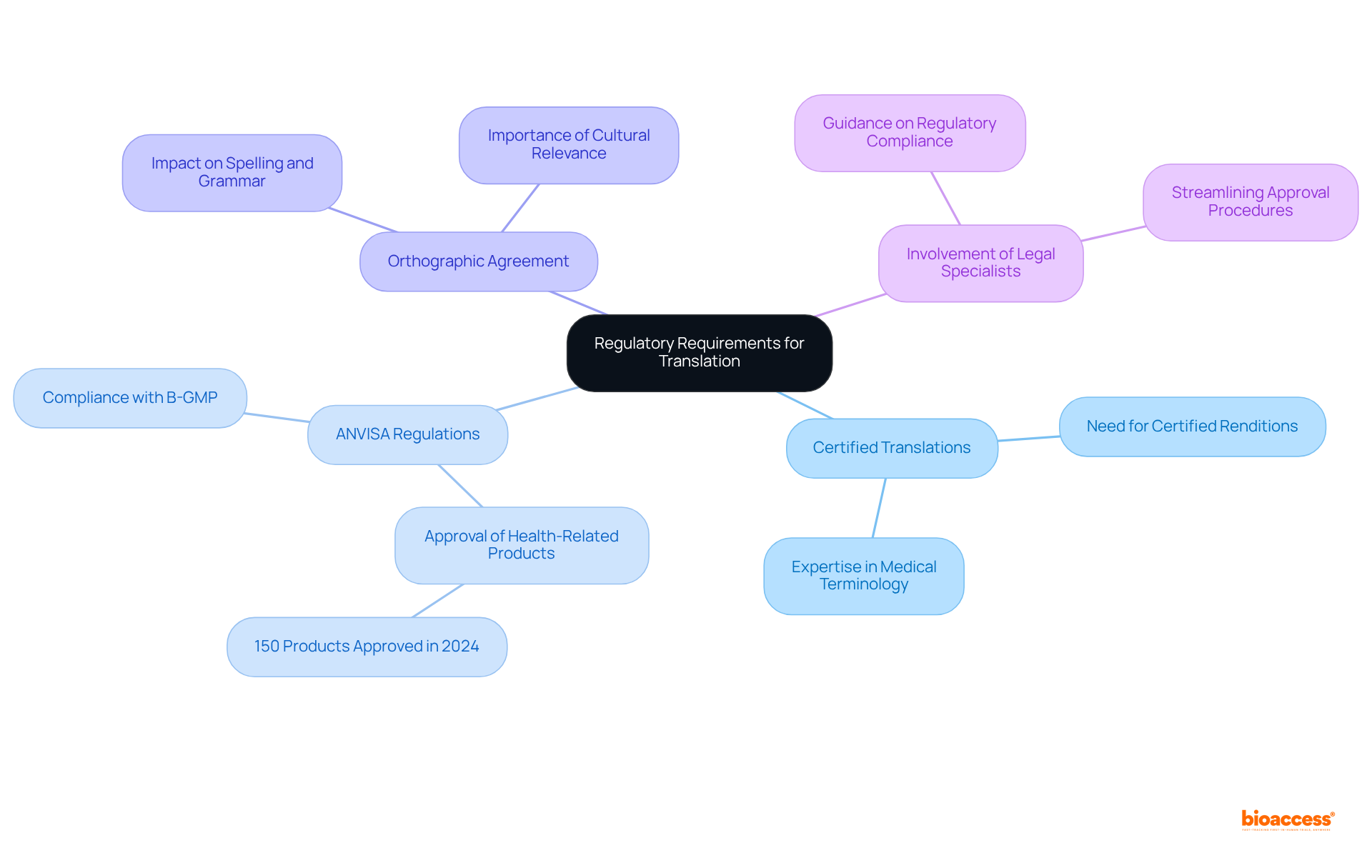
Navigating the complex landscape of technical file translation in Brazil requires more than mere linguistic skills; it demands a profound understanding of regulatory frameworks and industry standards. Companies aiming to ensure compliance with Brazilian laws and facilitate smoother approval processes for medical devices must prioritize certified translations that adhere to stringent requirements.
However, with evolving regulations and the critical need for accuracy, how can organizations effectively select qualified translators and maintain consistency in terminology? This article outlines four essential steps to streamline the process of purchasing technical file translation services in Brazilian Portuguese, ensuring both quality and compliance.
In Brazil, to ensure compliance with local laws, it is essential to purchase technical file translation Brazil Portuguese, as specialized document conversions must adhere to stringent regulatory requirements. A crucial element is the requirement for certified renditions, which are frequently mandated for legal and technical documents, particularly when you want to purchase technical file translation Brazil Portuguese.
Familiarity with the Brazilian National Health Surveillance Agency (ANVISA) regulations is essential for those who wish to purchase technical file translation Brazil Portuguese, as these regulations dictate the standards for medical device documentation. For instance, in 2024, ANVISA approved 150 health-related products, underscoring its commitment to innovation and public health.
Moreover, the Orthographic Agreement of the Portuguese Language affects spelling and grammar in adaptations, making it essential for companies to remain informed. Involving local legal specialists can offer important perspectives on these requirements, ensuring that all renditions not only comply with legal standards but are also recognized by regulatory authorities, thus enabling smoother approval procedures.
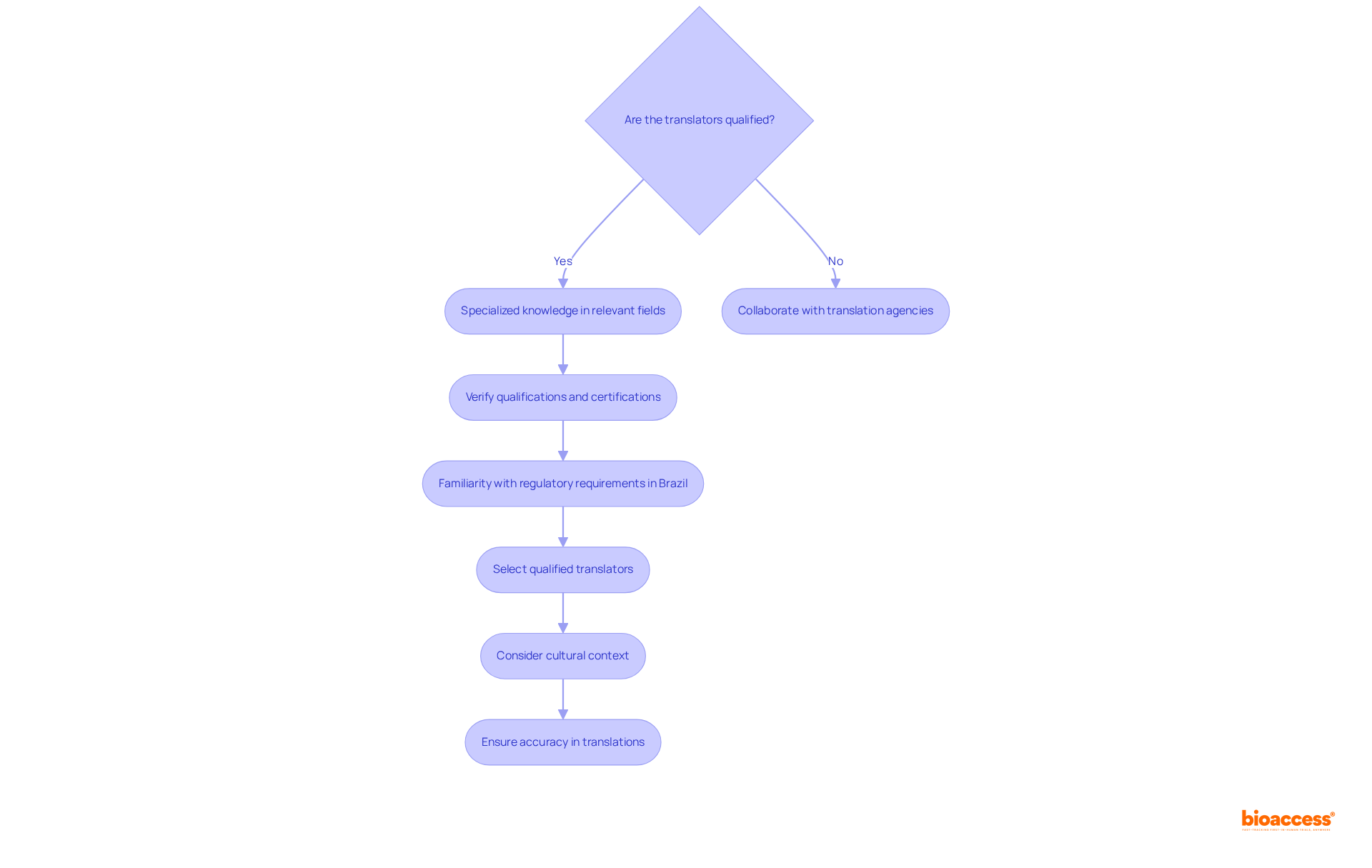
When selecting individuals for technical document conversions, it is essential to choose experts with specialized knowledge in the relevant field. Seek translators who have a background in medical technology, biopharmaceuticals, or radiopharmaceuticals, as their expertise will enable them to understand and accurately translate complex terminology. Verify their qualifications, including certifications in language services and relevant industry experience. Additionally, consider their familiarity with the specific regulatory requirements in Brazil, as this insight will enhance the quality and compliance of the purchase technical file translation Brazil Portuguese.
Collaborating with translation agencies that specialize in technical translations can also provide access to qualified professionals. Given that 80% of scientific papers are written in English, the ability to translate these documents precisely is vital for effective communication in the healthcare sector. Furthermore, understanding cultural context is crucial, as certain health-related terms may have different meanings in various countries, potentially leading to misunderstandings if not properly addressed.
With over 15 years of experience, bioaccess® emphasizes the importance of proficiency in medical language services, ensuring that selected professionals can adeptly navigate the complexities of medical terminology and regulatory standards. Avoid common pitfalls such as selecting language specialists without relevant experience or failing to verify their qualifications, as these mistakes can compromise the quality of the translated text and ultimately impact patient care.
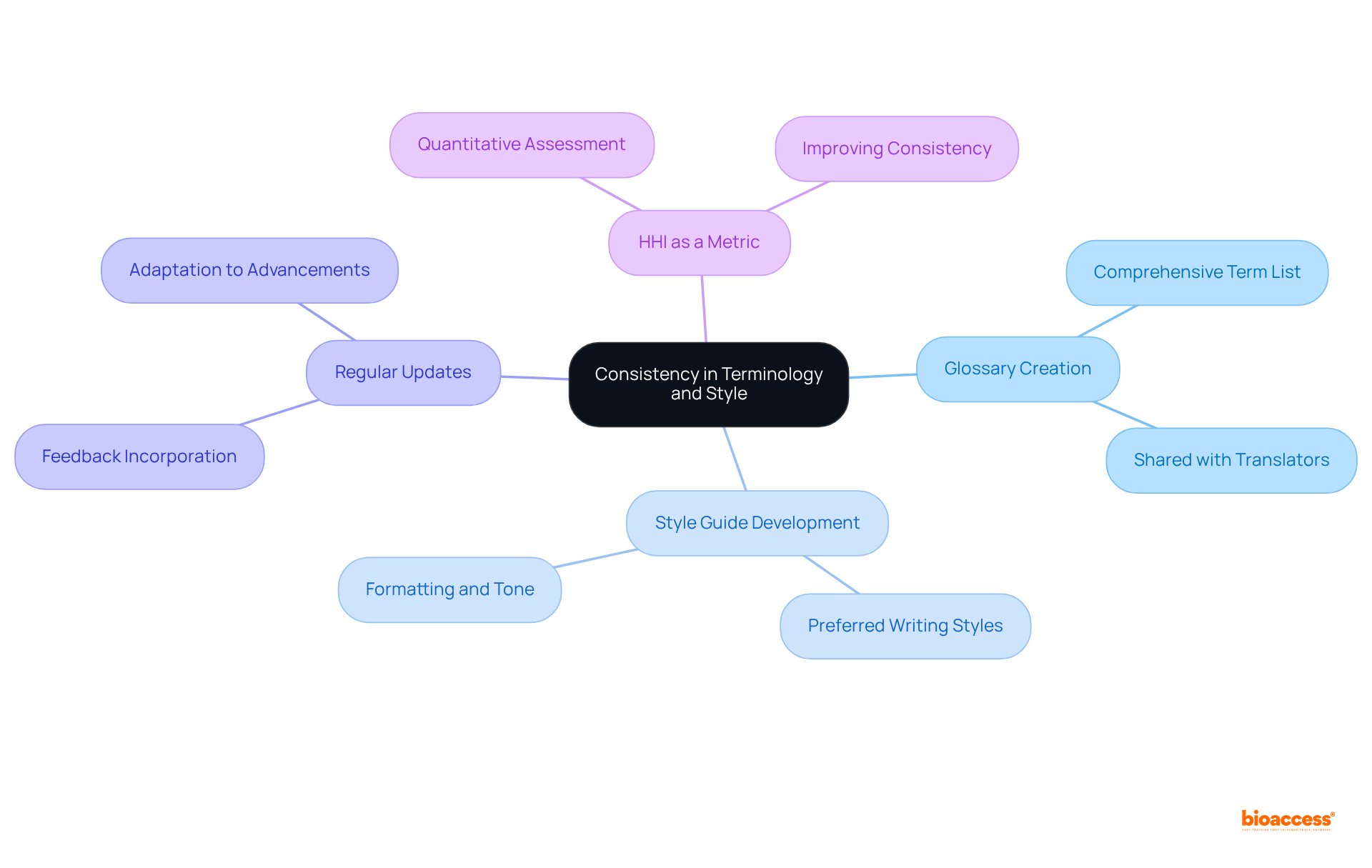
To achieve uniformity in terminology and style throughout specialized file adaptations, creating a comprehensive glossary of terms relevant to your sector is essential. This glossary must be shared with all translators involved in the project to guarantee that the same terms are utilized consistently across the documentation.
Research indicates that consistent terminology significantly reduces ambiguity and misunderstanding, making it a vital component of successful technical conversions. Furthermore, developing a style guide that delineates preferred writing styles, formatting, and tone will help sustain a cohesive voice across all translated materials.
Regularly updating these resources based on feedback and recent advancements in the field will enhance consistency and clarity in renditions. The Herfindahl-Hirshman Index (HHI) serves as a valuable metric for evaluating terminology consistency, providing a quantitative foundation for the importance of maintaining uniformity in rendered texts.
By prioritizing these practices, organizations can elevate the quality and effectiveness of their files in Brazil and beyond, especially with the increased demand for healthcare documents, which surged by 49% in 2020 due to COVID-19, leading to the need to purchase technical file translation Brazil Portuguese.
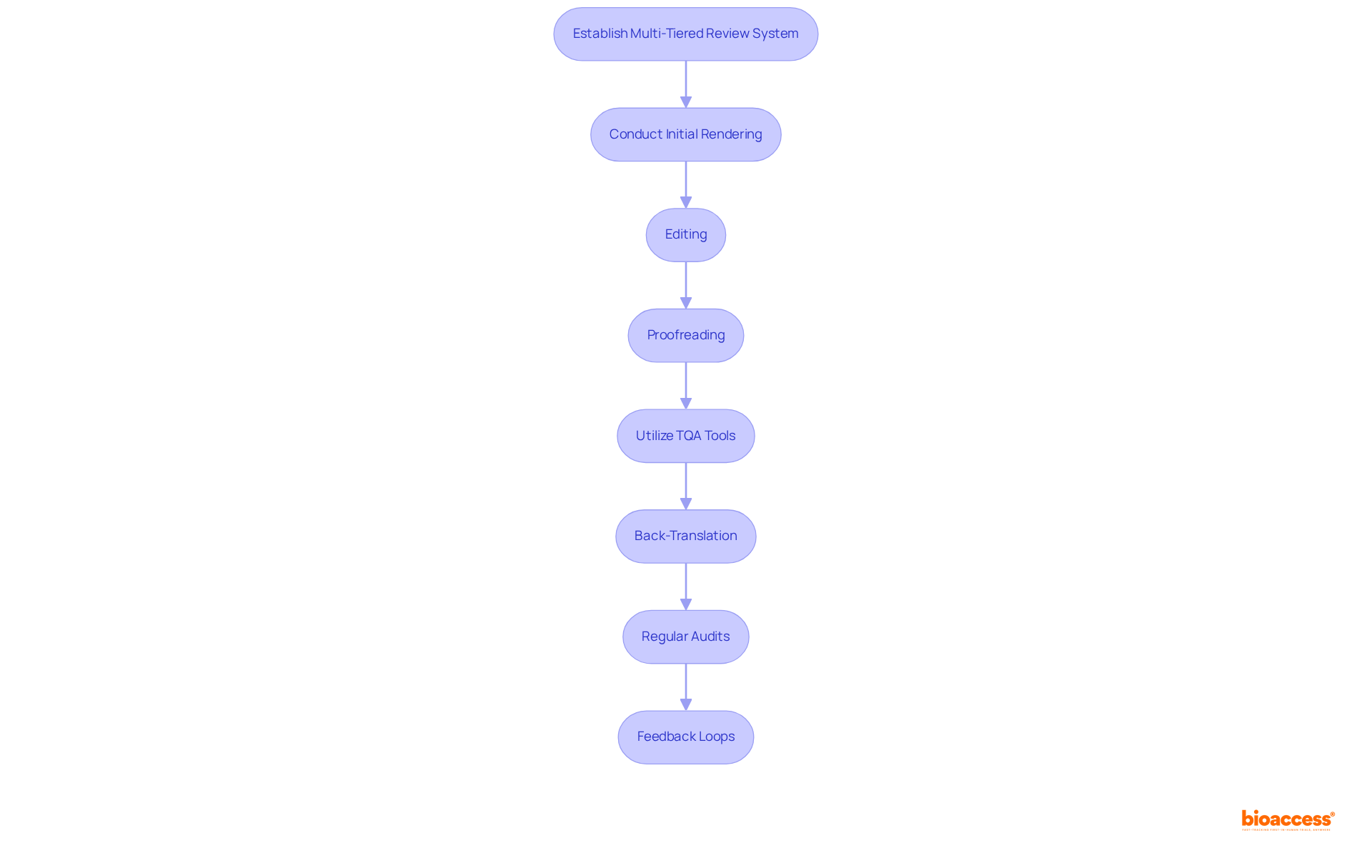
Implementing robust quality assurance processes for technical documentation is essential for ensuring accuracy and compliance in clinical research. Start by establishing a multi-tiered review system that encompasses initial rendering, editing, and proofreading stages. This structured approach facilitates multiple reviews, allowing for the identification and correction of errors or inconsistencies before final delivery.
Additionally, utilize quality assurance (TQA) tools to automate elements of the review process, such as ensuring terminology consistency and grammatical accuracy—both critical in medical documentation. Furthermore, consider employing back-translation, where a different individual translates the document back into the original language, serving as a verification method for accuracy.
Regular audits of the linguistic process, along with feedback loops involving translators, are vital for maintaining high-quality standards over time. This systematic approach not only enhances translation quality but also aligns with industry best practices, ultimately supporting the successful advancement of medical devices and biopharmaceuticals.
Purchasing technical file translation services in Brazil necessitates a comprehensive understanding of the regulatory landscape, specialized translator selection, consistency in terminology, and rigorous quality assurance processes. By prioritizing these crucial steps, organizations can ensure that their translations not only meet legal requirements but also effectively communicate complex information within the healthcare sector.
Key insights highlighted throughout the article underscore the necessity of:
Furthermore, implementing quality assurance measures is vital for safeguarding accuracy and compliance in translated documents, ultimately supporting the successful introduction of medical devices and biopharmaceuticals to the Brazilian market.
In conclusion, the significance of adhering to these best practices cannot be overstated. Organizations aiming to purchase technical file translations in Brazilian Portuguese should actively engage in thorough research and collaboration with qualified professionals. By doing so, they not only enhance the quality and effectiveness of their documentation but also contribute to improved patient care and regulatory success in Brazil.
Why is it important to understand regulatory requirements for Brazilian Portuguese translation?
Understanding regulatory requirements is essential to ensure compliance with local laws, particularly for technical file translations, which must adhere to stringent standards.
What kind of translations require certification in Brazil?
Legal and technical documents, especially those related to medical devices, often require certified translations to meet regulatory standards.
What organization’s regulations should be familiarized with for technical file translation in Brazil?
Familiarity with the Brazilian National Health Surveillance Agency (ANVISA) regulations is crucial, as they dictate the standards for medical device documentation.
Can you provide an example of ANVISA's recent activity?
In 2024, ANVISA approved 150 health-related products, highlighting its commitment to innovation and public health.
How does the Orthographic Agreement of the Portuguese Language impact translations?
The Orthographic Agreement affects spelling and grammar in translations, making it important for companies to stay informed about these changes.
Why is it beneficial to involve local legal specialists in the translation process?
Involving local legal specialists can provide important insights into regulatory requirements, ensuring compliance and smoother approval procedures with regulatory authorities.