


The article outlines four strategies for effective Good Clinical Practice (GCP) training in clinical research, emphasizing the enhancement of training efficiency, compliance assurance, and the adoption of continuous improvement practices. It highlights the critical role of:
in fostering a deep understanding and retention of GCP principles. These principles are essential for maintaining regulatory compliance and improving research outcomes, making them a pivotal focus in the clinical research landscape.
The landscape of clinical research is continually evolving, underscoring the critical importance of Good Clinical Practice (GCP) training. This foundational training not only protects the rights and well-being of trial participants but also guarantees the integrity and reliability of research outcomes. As organizations strive for compliance with stringent regulatory standards, the challenge lies in implementing effective training strategies that adapt to the latest methodologies and technologies.
How can clinical research teams enhance their GCP training to meet compliance requirements while fostering a culture of continuous improvement and engagement?
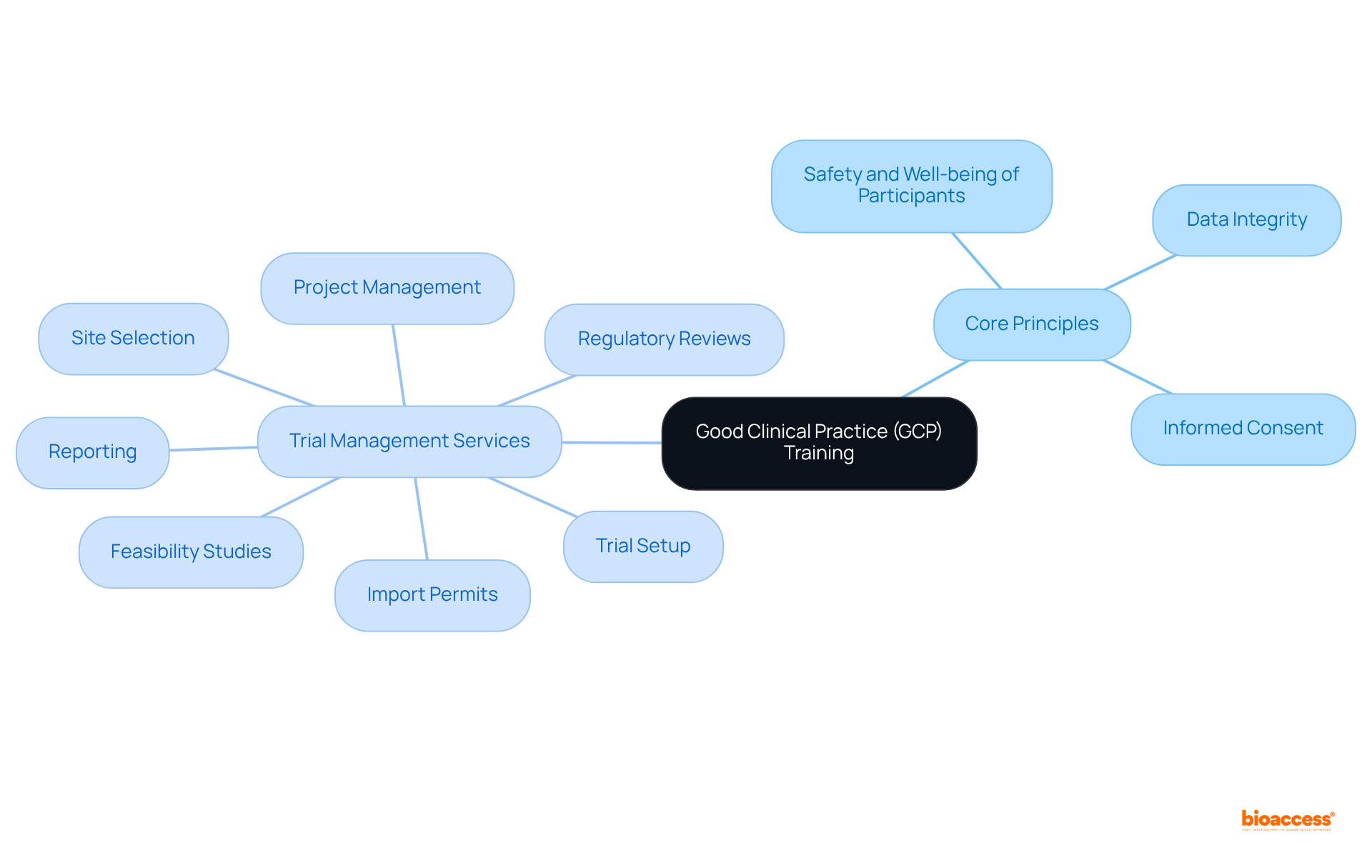
Good Clinical Practice (GCP) represents an international ethical and scientific quality standard essential for the design, conduct, recording, and reporting of trials involving human subjects. It is imperative to understand GCP by familiarizing oneself with its core principles, which include:
Training must encompass the responsibilities of investigators, sponsors, and monitors, alongside the critical importance of adhering to regulatory requirements established by organizations such as the FDA and EMA.
Thorough trial management services—comprising:
are crucial in enhancing GCP training. These services not only facilitate adherence to GCP standards but also enhance the overall efficiency of research management. By establishing a solid foundation in GCP, clinical research teams can ensure compliance and significantly enhance the credibility of their research outcomes.
To enhance the efficiency of GCP training, organizations must adopt several strategic approaches.
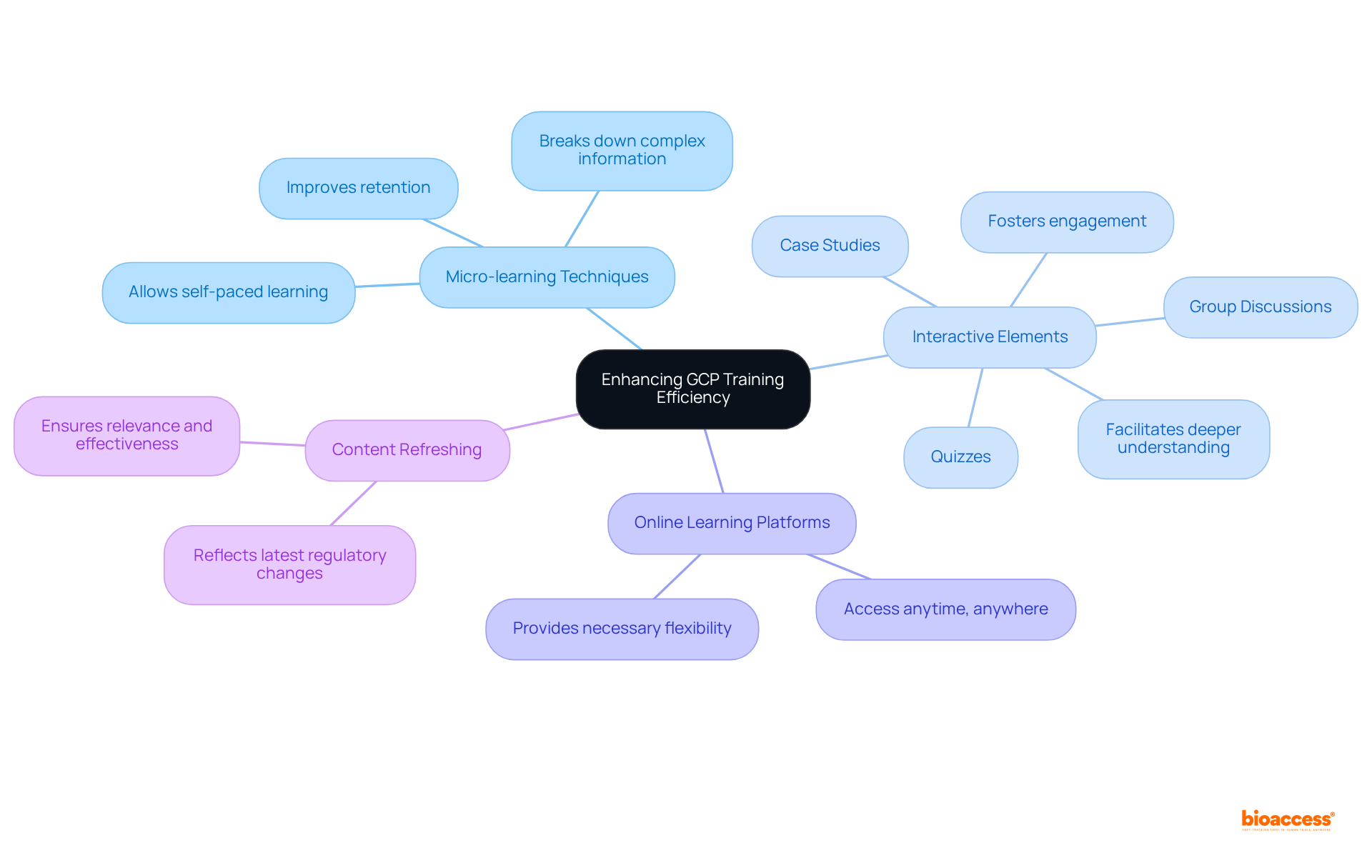
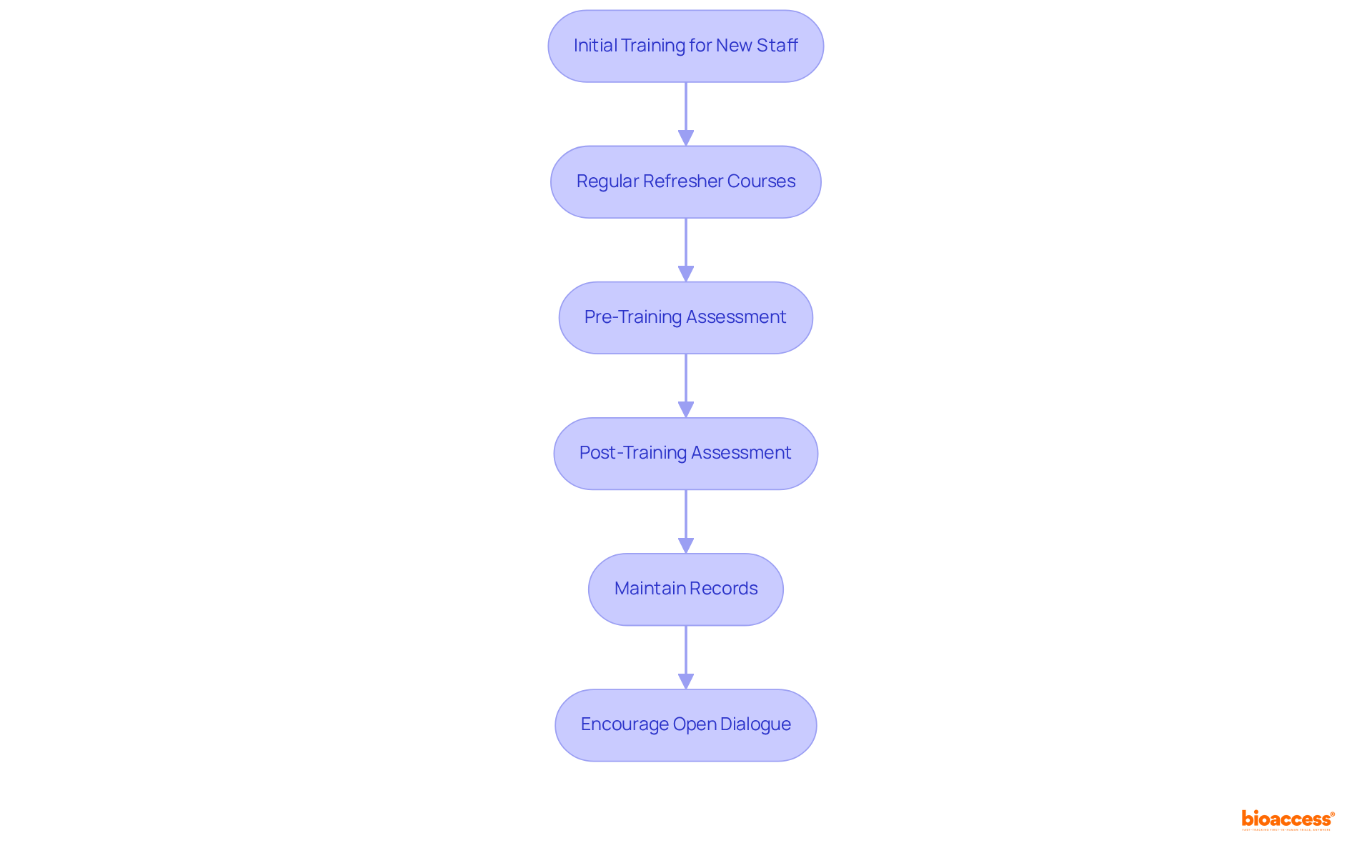
Effective GCP training methods are essential for ensuring compliance with regulatory standards in clinical research. Organizations must establish a comprehensive development program that encompasses initial training for new staff and regular refresher courses for current personnel.
By implementing pre- and post-training assessments, organizations can effectively gauge understanding and retention of GCP principles. Furthermore, maintaining detailed records of educational activities is critical for demonstrating adherence during evaluations.
Encouraging open dialogue regarding compliance issues and providing resources for continuous education can significantly enhance a culture of conformity within the organization.
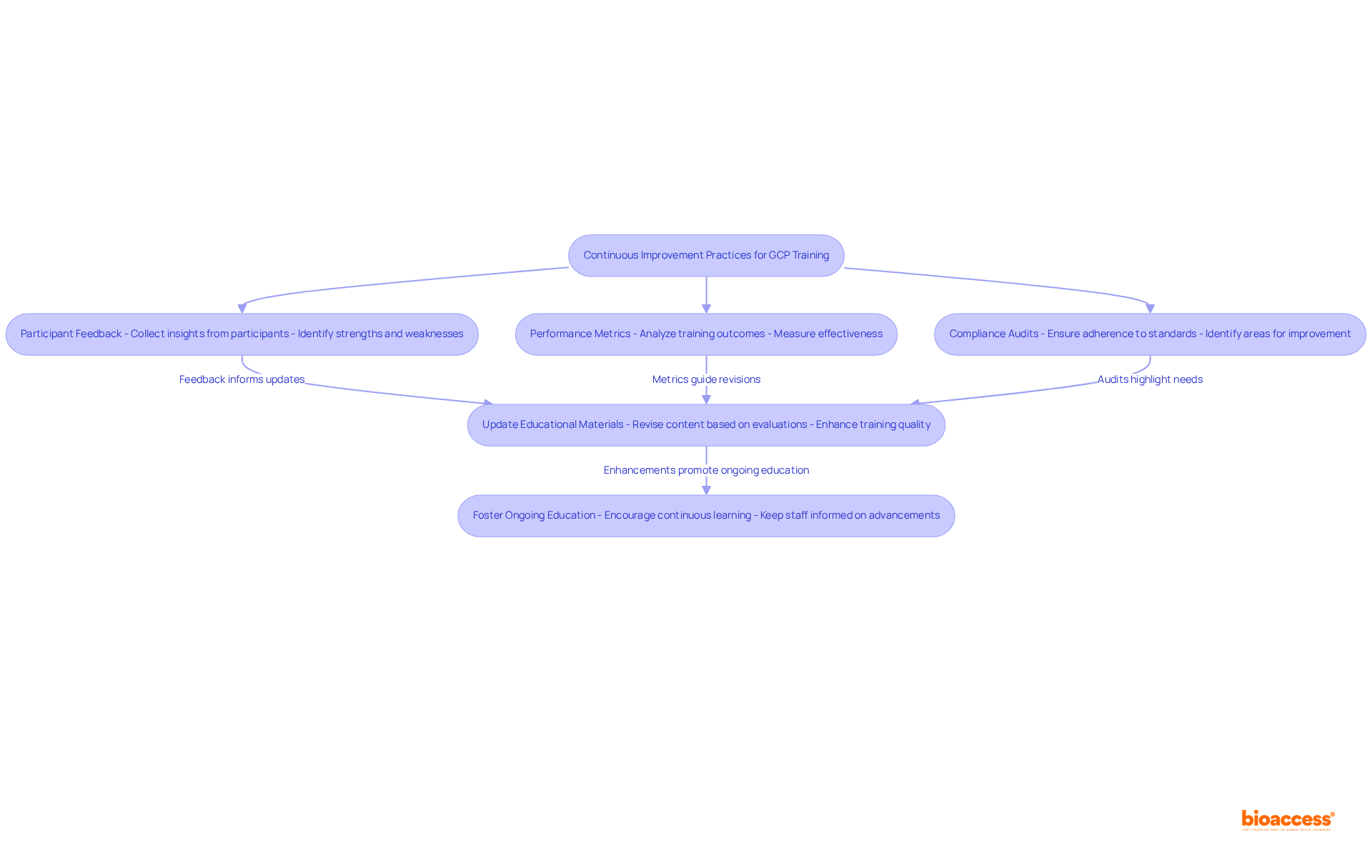
Continuous improvement practices must be an integral component of GCP educational programs. Organizations are urged to regularly assess the effectiveness of their programs through:
This evaluation process not only identifies areas for enhancement but also informs necessary updates to educational materials. Furthermore, fostering a culture of ongoing education serves to motivate staff to stay informed about the latest advancements in GCP and research. By adopting a continuous improvement mindset, organizations can significantly enhance the quality of their GCP training, ensuring that their teams are well-prepared to address the challenges of clinical research.
Implementing effective GCP training strategies is crucial for clinical research organizations striving to uphold the highest ethical and scientific standards. A robust understanding of Good Clinical Practice not only safeguards participant welfare and data integrity but also enhances the credibility of research outcomes. By focusing on comprehensive training methodologies that incorporate innovative techniques, organizations can ensure their teams are well-equipped to navigate the complexities of clinical trials.
The article highlights several key strategies for enhancing GCP training efficiency, including:
These approaches foster deeper engagement with the material and promote better retention of essential GCP principles. Additionally, establishing a comprehensive development program, conducting assessments, and maintaining thorough educational records are vital for ensuring compliance with regulatory standards.
Ultimately, the pursuit of continuous improvement in GCP training practices cannot be overstated. By actively seeking participant feedback, analyzing performance metrics, and conducting compliance audits, organizations can identify areas for enhancement and adapt their training programs accordingly. Embracing a culture of ongoing education not only motivates staff to stay informed about the latest advancements but also reinforces the significance of effective GCP training in achieving regulatory compliance and advancing clinical research.
What is Good Clinical Practice (GCP)?
Good Clinical Practice (GCP) is an international ethical and scientific quality standard essential for the design, conduct, recording, and reporting of trials involving human subjects.
Why is it important to understand GCP?
Understanding GCP is crucial for ensuring the safety and well-being of trial participants, obtaining informed consent, and maintaining data integrity in clinical research.
What are the core principles of GCP?
The core principles of GCP include ensuring the safety and well-being of trial participants, obtaining informed consent, and maintaining data integrity.
Who needs to be trained in GCP?
Training in GCP is necessary for investigators, sponsors, and monitors involved in clinical trials.
What regulatory organizations establish GCP requirements?
Regulatory requirements for GCP are established by organizations such as the FDA (Food and Drug Administration) and EMA (European Medicines Agency).
What are some key components of trial management services that enhance GCP training?
Key components of trial management services include feasibility studies, site selection, regulatory reviews, trial setup, import permits, project management, and reporting.
How do trial management services contribute to GCP training?
Trial management services facilitate adherence to GCP standards and enhance the overall efficiency of research management.
What is the benefit of establishing a solid foundation in GCP?
Establishing a solid foundation in GCP helps clinical research teams ensure compliance and significantly enhances the credibility of their research outcomes.