


The article titled "5 Key Insights from RAPS 2023 for Clinical Research Directors" presents essential strategies that clinical research directors must consider to effectively navigate the evolving landscape of clinical research and regulatory compliance. It underscores the necessity of proactive engagement with regulatory authorities, continuous education on IVDR requirements, and collaboration with experienced partners like bioaccess® to enhance compliance and streamline research processes.
In today’s Medtech landscape, understanding the intricacies of regulatory compliance is crucial. Clinical research directors face numerous challenges, from adapting to new regulations to ensuring that their teams are well-informed. By partnering with bioaccess®, organizations can leverage their expertise to address these challenges effectively. This collaboration not only fosters compliance but also enhances the overall efficiency of research processes.
Ultimately, the importance of collaboration cannot be overstated. As clinical research continues to evolve, staying ahead of regulatory changes and industry trends is vital. Engaging with knowledgeable partners like bioaccess® can provide the necessary support to navigate these complexities. Clinical research directors are encouraged to take proactive steps towards building these essential partnerships.
As the landscape of clinical research evolves, the insights gleaned from RAPS 2023 present a crucial opportunity for Clinical Research Directors to refine their strategies. This article explores five key takeaways that underscore the importance of navigating regulatory complexities, enhancing documentation practices, and fostering collaboration to ensure compliance with the In Vitro Diagnostic Regulation (IVDR). With increasing demands for high-quality evidence and the challenges of adapting to new regulations, how can directors effectively position their organizations for success in this competitive environment?
The Medtech landscape is shifting rapidly, and understanding these dynamics is essential for success. By addressing the key challenges highlighted in this article, Clinical Research Directors can not only meet regulatory requirements but also drive innovation within their organizations. The need for collaboration and strategic planning has never been more critical.
In summary, the insights from RAPS 2023 serve as a roadmap for Clinical Research Directors. By embracing these takeaways, they can navigate the complexities of the regulatory environment and position their organizations for future success.
bioaccess® leverages its extensive expertise in early-stage research to streamline the regulatory compliance process. By capitalizing on its geographical advantages, the organization facilitates rapid ethical approvals and accelerates patient enrollment, ensuring alignment with the stringent criteria set forth by the IVDR. This strategy not only shortens timelines but also elevates the quality of research evidence necessary for submissions to regulatory authorities.
Industry leaders emphasize that robust ethical approvals are crucial for the success of research studies, positioning bioaccess® as a vital partner for Medtech and Biopharma innovators navigating these complexities. The ability to secure ethical approvals in just 4-6 weeks significantly enhances the likelihood of timely market entry, empowering companies to maintain a competitive edge in an evolving compliance landscape.

Producers face considerable challenges in recording evidence from trials, primarily due to variations in data gathering techniques and the necessity of adhering to industry standards. This inconsistency can lead to delays in product approvals and increased costs. To address these issues, Clinical Research Directors must prioritize the establishment of standardized protocols for data collection and invest in comprehensive training for research teams. This ensures compliance with both these protocols and legal requirements.
Moreover, utilizing advanced data management systems can significantly streamline the documentation process, enhancing both accuracy and efficiency. By implementing these strategies, organizations can improve their evidence documentation and facilitate smoother compliance approvals.
Collaboration with bioaccess®—which specializes in comprehensive trial management services, including Early-Feasibility Studies, First-In-Human Studies, and Post-Market Follow-Up Studies—enables organizations to effectively navigate the complexities of trials in Latin America. This partnership can lead to improved documentation processes and overall success in clinical research.
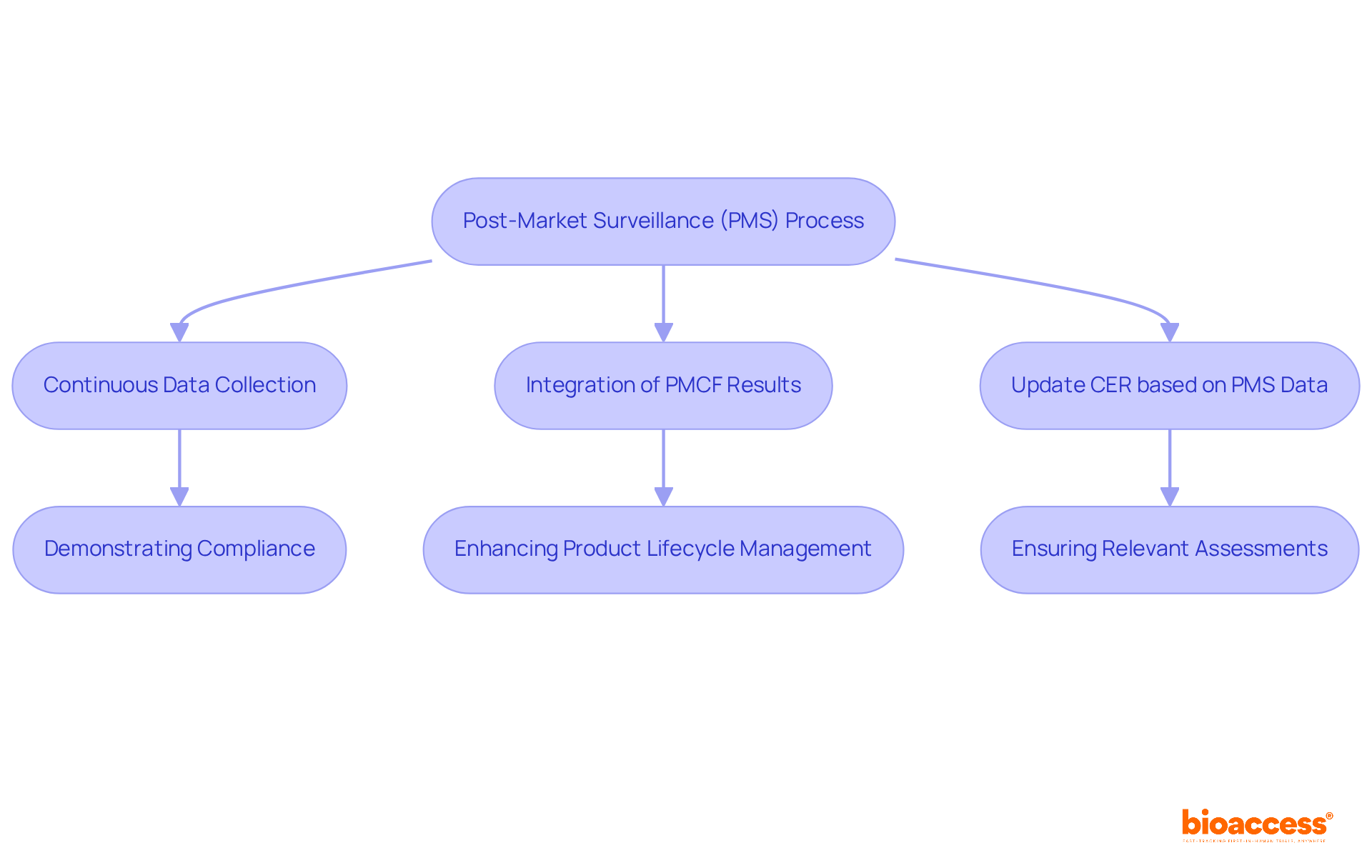
The Post-Market Surveillance (PMS) process is crucially aligned with Clinical Evaluation Reports (CER), both emphasizing the need for continuous data collection and analysis to ensure product safety and efficacy. A systematic approach to gathering medical evidence is essential for demonstrating compliance with industry standards. For example, integrating results from post-market follow-up (PMCF) studies—a key area of expertise for bioaccess®—into CERs significantly impacts the risk/benefit profile and performance assessments.
Regulatory guidelines mandate that the CER be updated based on PMS data, including PMCF results, to reflect the latest information on device safety and effectiveness. Research Directors must prioritize training their teams in both processes, as effectively integrating PMS into the overall strategy can enhance product lifecycle management and regulatory compliance. Continuous data collection not only supports ongoing safety evaluations but also meets regulatory expectations, ensuring that assessments remain relevant and indicative of real-world device performance.
As industry experts highlight, understanding a medical product’s safety profile evolves as safety data accumulates, emphasizing the need for a proactive approach to data management. By leveraging bioaccess®'s 20+ years of experience in managing Early-Feasibility Studies (EFS), First-In-Human Studies (FIH), Pilot Studies, Pivotal Studies, and Post-Market Follow-Up Studies, organizations can significantly bolster these efforts.
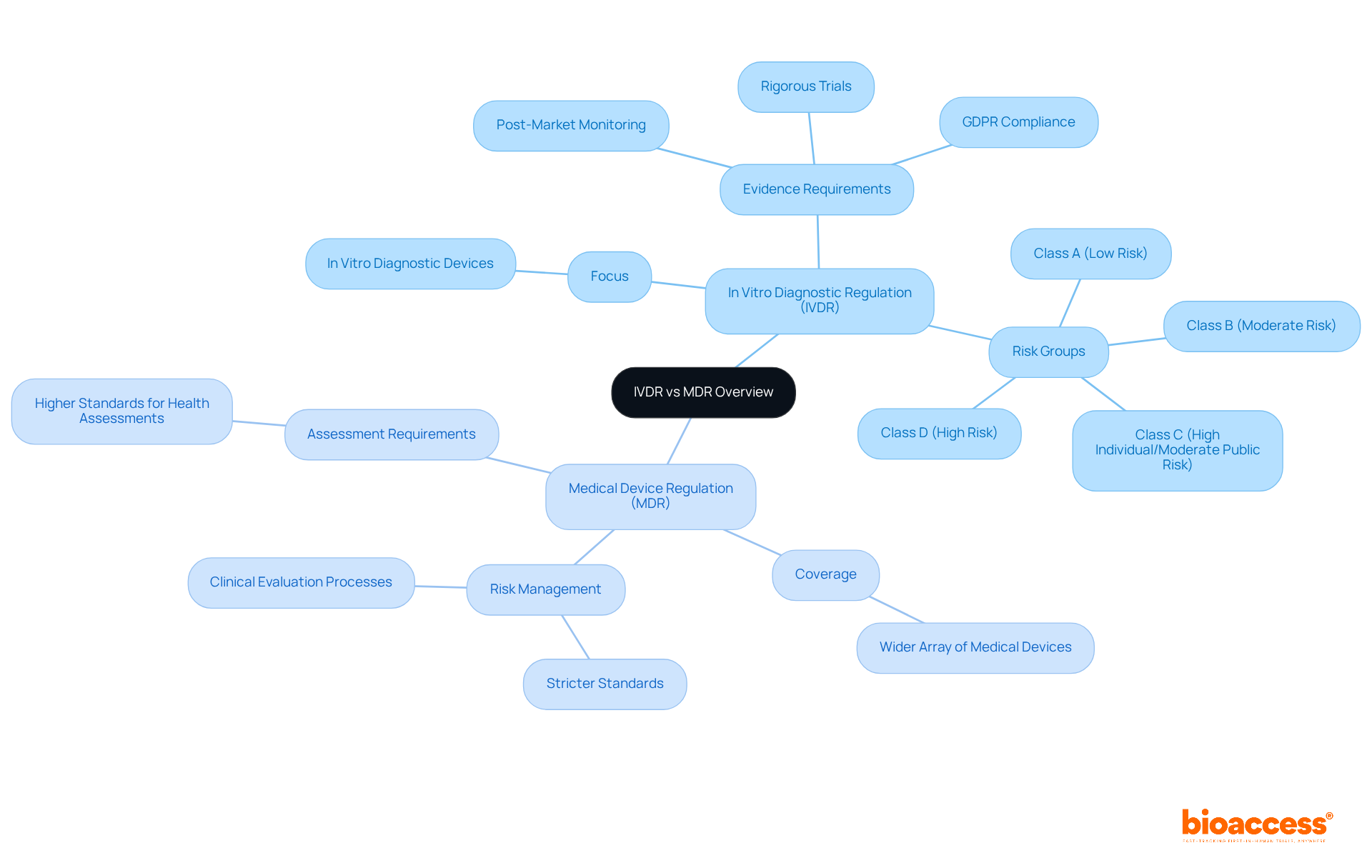
The In Vitro Diagnostic Regulation and the Medical Device Regulation are crucial for ensuring the safety and effectiveness of medical products, yet they differ significantly in their focus and requirements. The In Vitro Diagnostic Regulation specifically targets in vitro diagnostic devices, mandating rigorous evidence from trials and comprehensive post-market monitoring to enhance patient safety and product efficacy. Conversely, the Medical Device Regulation (MDR) covers a wider array of medical devices, imposing stricter standards for health assessments and risk management processes.
Clinical Research Directors must adeptly navigate these differences to tailor their research strategies effectively. With the new regulations, the emphasis is on generating high-quality medical evidence that meets updated regulatory standards, which include meticulous documentation and compliance with GDPR for patient privacy. This is in contrast to the MDR, which necessitates a broader scope of risk management and clinical evaluation processes.
For instance, the In Vitro Diagnostic Regulation classifies diagnostic tests into four risk groups (A, B, C, and D), each with increasing requirements for notified body involvement. In comparison, the MDR encompasses a wider range of devices with its own classification framework. As a result, approximately 80% of tests under the new regulation now require assessment by notified bodies, a significant increase from the previous rules where only about 10% faced such scrutiny.
Regulatory professionals stress the importance of grasping these distinctions. Industry experts highlight that the stringent standards of the regulation aim to elevate the overall quality and safety of in vitro diagnostics, ultimately enhancing patient care. Therefore, Clinical Research Directors must ensure their research strategies align with the specific requirements of either the In Vitro Diagnostic Regulation or the MDR, depending on their products, to maintain compliance and optimize clinical outcomes.
Insights from raps 2023 underscore the critical need for proactive engagement with oversight authorities and highlight the importance of continuous education regarding the evolving requirements of IVDR. Clinical Research Directors must cultivate a culture of adherence within their organizations, ensuring that all team members are well-informed and equipped with the latest tools to navigate the regulatory landscape effectively.
Participating in ongoing training programs has proven to enhance adherence effectiveness, with organizations reporting improved conformity to regulations. Customized training programs tailored to specific roles within the research team are essential for effective regulation management. Furthermore, collaboration with knowledgeable partners like bioaccess® can provide vital insights and support, facilitating adherence and expediting research timelines.
bioaccess® offers comprehensive trial management services, including:
As Katherine Ruiz, a specialist in compliance affairs for medical devices and in vitro diagnostics in Colombia, emphasizes, "Collaboration with bioaccess® not only addresses current challenges but also positions the sponsor of clinical trials for success in a competitive landscape."
This strategic approach not only strengthens regulatory relationships but also positions organizations for success in an increasingly complex research environment. By prioritizing collaboration and continuous education, clinical research teams can navigate the evolving landscape with confidence.
Insights from RAPS 2023 underscore the urgent need for Clinical Research Directors to adapt to the shifting regulatory landscape, especially concerning the In Vitro Diagnostic Regulation (IVDR). The discussions highlighted the critical importance of proactive engagement with regulatory authorities and fostering a culture of compliance within organizations. By prioritizing continuous education and collaborating with experienced partners like bioaccess®, research teams can navigate these complexities more effectively.
The article outlines several key strategies, including:
These elements are vital for streamlining documentation processes, securing ethical approvals swiftly, and ensuring that clinical evidence meets the stringent standards set by regulatory bodies. The focus on integrating post-market surveillance data into clinical evaluation reports further emphasizes the need for a systematic approach to data management.
In conclusion, the insights from RAPS 2023 serve as a clarion call for Clinical Research Directors to adopt a proactive and informed stance on regulatory compliance. By investing in education, standardizing processes, and collaborating with specialized partners, organizations can not only enhance their compliance efforts but also position themselves for success in a competitive and ever-evolving research environment. The path forward is clear: prioritize collaboration, continuous learning, and strategic planning to navigate the complexities of clinical research and ensure the delivery of safe and effective medical products to patients.
What is bioaccess® and what role does it play in clinical research?
bioaccess® leverages its expertise in early-stage research to streamline the regulatory compliance process, facilitating rapid ethical approvals and accelerating patient enrollment for Medtech and Biopharma innovators.
How does bioaccess® enhance the regulatory compliance process?
bioaccess® enhances the regulatory compliance process by securing ethical approvals in just 4-6 weeks, which shortens timelines and elevates the quality of research evidence necessary for submissions to regulatory authorities.
Why are robust ethical approvals important for research studies?
Robust ethical approvals are crucial for the success of research studies as they ensure compliance with ethical standards, thereby increasing the likelihood of timely market entry and helping companies maintain a competitive edge.
What challenges do manufacturers face in documenting clinical evidence?
Manufacturers face challenges in documenting clinical evidence due to variations in data gathering techniques and the need to adhere to industry standards, which can lead to delays in product approvals and increased costs.
What strategies can Clinical Research Directors implement to improve evidence documentation?
Clinical Research Directors should establish standardized protocols for data collection, invest in comprehensive training for research teams, and utilize advanced data management systems to enhance accuracy and efficiency in documentation.
How can collaboration with bioaccess® benefit organizations in clinical research?
Collaboration with bioaccess® can help organizations navigate the complexities of trials in Latin America, leading to improved documentation processes and overall success in clinical research through their comprehensive trial management services.