

Thorough QT (TQT) studies are essential in clinical research, acting as a vital safeguard in the development of new medications. These specialized investigations evaluate the effects of drugs on the QT interval, a crucial indicator of cardiac safety. By identifying potential risks early in the drug development process, TQT studies play a pivotal role in ensuring patient safety and drug efficacy.
However, as methodologies evolve and regulatory landscapes shift, clinical research leaders face the challenge of navigating the complexities of TQT studies. How can they enhance patient safety while ensuring the effectiveness of new drugs? Understanding the nuances of TQT and its implications provides critical insights that can shape the future of medication development.
In this context, collaboration and innovative approaches are paramount. By leveraging advancements in research and technology, stakeholders can address key challenges in the Medtech landscape, ultimately leading to safer and more effective therapeutic options.
Thorough QT (TQT) investigations are specialized clinical trials that evaluate the potential effects of new medications on the QT interval of the heart's electrical cycle, which relates to the tqt meaning, specifically the QTc interval. These investigations are crucial in medication development, as they help identify any proarrhythmic hazards associated with new chemical entities (NCEs). Typically, a TQT examination employs a randomized, double-blind, placebo-controlled design, where participants receive either the investigational medication or a placebo, with their heart rhythms monitored through electrocardiograms (ECGs). The primary objective is to determine whether the medication extends the QTc interval by 10 milliseconds or more, a threshold that raises regulatory concerns.
In recent years, the number of TQT investigations has remained stable, with standalone TQT assessments accounting for approximately 50% of all data reports reviewed by the FDA. For instance, moxifloxacin has frequently served as a positive control in these investigations, demonstrating a peak effect of around 14 milliseconds. This underscores the importance of thorough QT evaluations in safety assessments.
The significance of TQT research, specifically its tqt meaning, in medication advancement cannot be overstated. These studies not only enhance our understanding of the pharmacological effects on the QT/QTc interval but also provide valuable pharmacokinetic information that can enrich the overall therapeutic portfolio. Industry specialists emphasize that conducting TQT assessments early in the development process can mitigate risks associated with the ongoing progress of a compound. Conversely, subsequent evaluations may be necessary if proof-of-concept efficacy data is required to justify the substantial costs involved, which can exceed $5 million. This strategic approach ensures that potential cardiac risks are thoroughly evaluated, ultimately contributing to safer therapeutic options for patients.
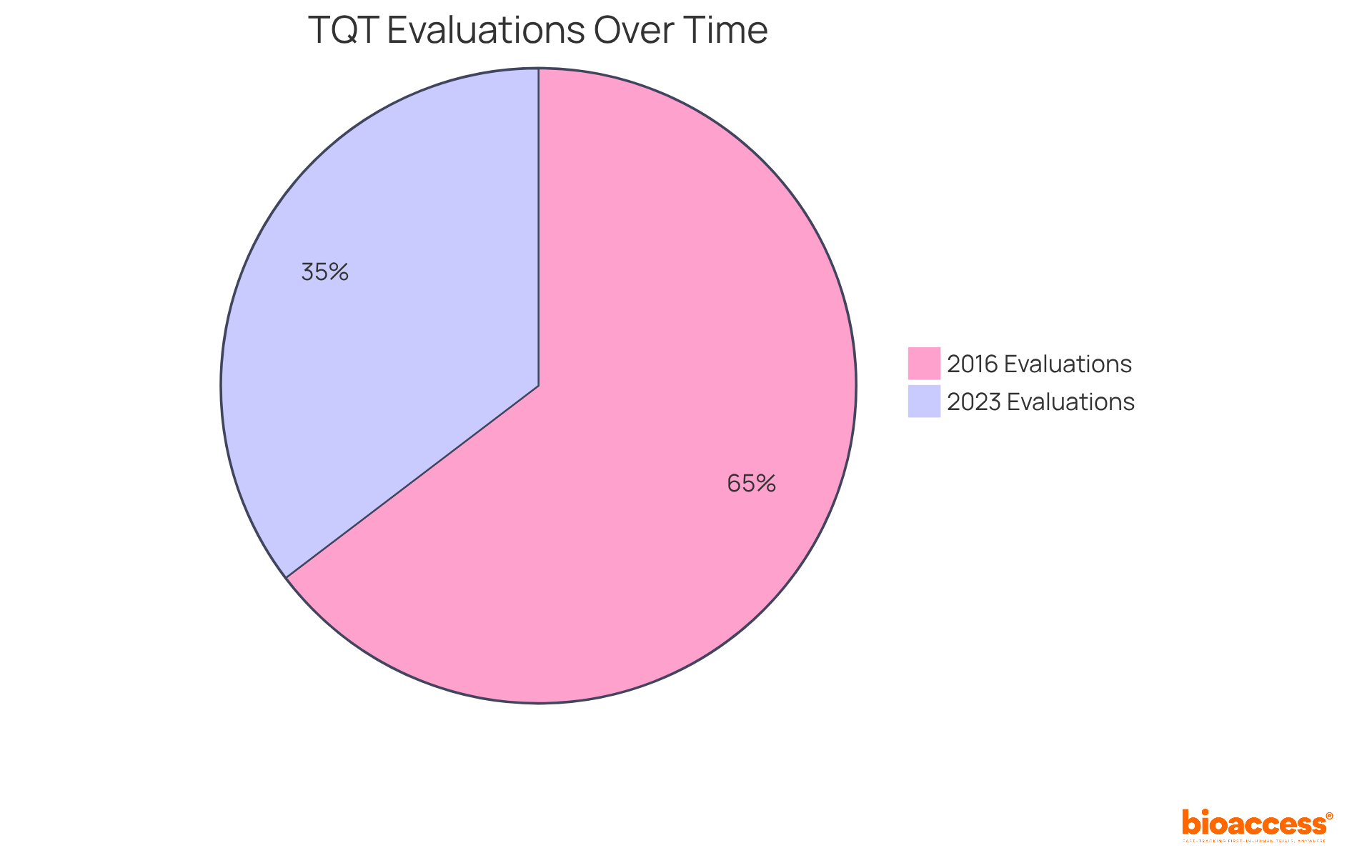
Thorough QT (TQT) investigations play a pivotal role in the clinical research landscape, as their tqt meaning delivers essential data on the cardiac safety of new drugs. Regulatory bodies, including the FDA and EMA, mandate examinations to understand tqt meaning for all new chemical entities (NCEs) with systemic exposure to assess the risk of arrhythmias. Notably, the percentage of TQT evaluations has decreased from 75% in 2016 to 41% in 2023, signaling a shift in how cardiac safety assessments are approached. Despite this decline, the meaning of TQT analyses still accounts for approximately 50% of all data reports evaluated by the FDA in recent years, underscoring their ongoing importance in the regulatory process.
The outcomes of these investigations can profoundly influence the medication development process. A negative TQT meaning typically allows for normal progression to subsequent clinical trial stages, while a positive result necessitates further ECG monitoring and may alter the drug's development pathway. For instance, the advancement of JNJ-54861911 highlighted the importance of elevated concentrations in C-QTc analysis. Initial examinations showed no significant QTc prolongation at lower doses, but increased doses revealed QTc prolongation, confirmed in a fully powered TQT evaluation. This example illustrates the critical nature of TQT studies and their tqt meaning in safeguarding patient health and ensuring that new therapies are both effective and safe.
As the landscape evolves, expert opinions suggest a transition towards smaller trials that effectively prevent torsadogenic substances from entering the market, reflecting a positive trend in cardiac safety assessments. Dr. Garnett from the FDA emphasizes that the standard for labeling has been that if an effect exceeding 10 milliseconds can be ruled out, it seems unlikely that the medication will cause a QTc effect surpassing 20 milliseconds. This insight reinforces the necessity of understanding tqt meaning in the ongoing quest for safer and more effective medical therapies.
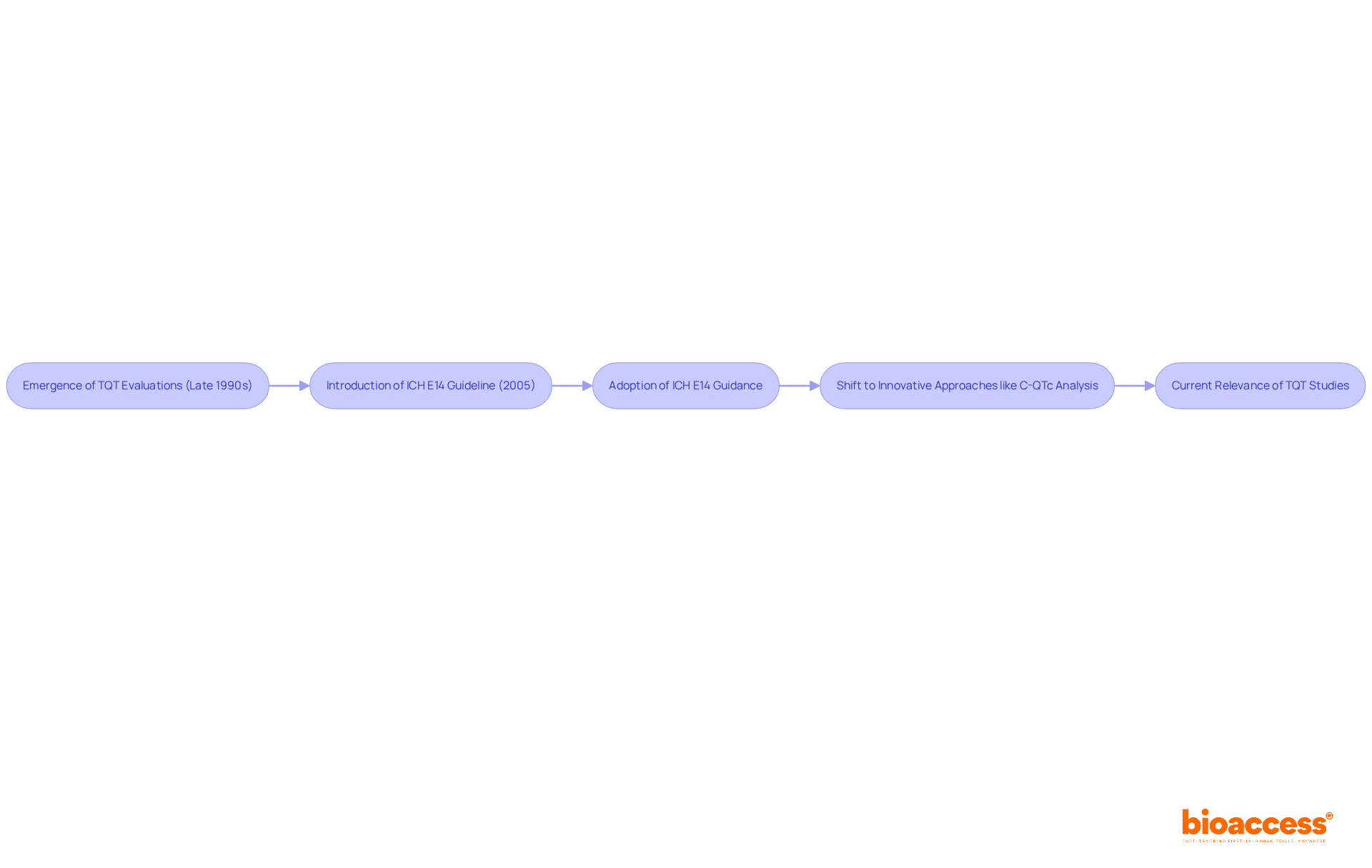
The concept of Thorough QT (TQT) evaluations emerged as a crucial response to the need for stringent cardiac safety assessments in drug development, particularly following significant instances of substance-induced arrhythmias in the late 1990s and early 2000s, which underscores the TQT meaning in the context of drug safety.
In 2005, the International Conference on Harmonisation (ICH) introduced the E14 guideline, which established a standardized framework for understanding TQT meaning in studies. This guideline played a pivotal role in shaping the regulatory landscape, underscoring the importance of assessing a drug's potential to prolong the QT interval, which relates to the TQT meaning as a critical indicator of cardiac safety.
As Borje Darpo noted, 'The ICH E14 guidance on how to clinically assess a new drug's liability to prolong the QT interval was adopted in May 2005.'
Over the years, the methodology and regulatory expectations surrounding TQT investigations have evolved significantly, reflecting the changing TQT meaning. Notably, there has been a shift towards innovative approaches, such as concentration-QTc (C-QTc) analysis, which allows for the evaluation of cardiac safety without necessitating a separate TQT examination.
This evolution mirrors a broader trend within the pharmaceutical industry towards more efficient, adaptive, and patient-centered drug development processes, ultimately enhancing the safety and efficacy of new therapeutics.
Importantly, independent analyses of TQT meaning still account for approximately 50% of all data reports reviewed by the FDA, underscoring their ongoing relevance in the current regulatory environment.
The tqt meaning highlights the crucial role of TQT investigations in clinical research, defined by their stringent design. Typically, these studies incorporate a crossover format, allowing participants to receive both the investigational compound and a placebo at various intervals. This method not only enhances the reliability of the results but also ensures that the findings are robust and replicable.
Key methodologies in TQT studies include:
As the Medtech landscape evolves, advancements in technology and data analysis techniques are continuously integrated into TQT studies. This integration not only enhances the efficiency of the investigations but also addresses key challenges faced in clinical research. By leveraging these innovations, researchers can better navigate the complexities of drug effects and patient responses.
In summary, TQT investigations are vital for understanding tqt meaning in ensuring the safety and efficacy of new compounds. Collaboration among researchers, regulatory bodies, and technology providers is essential to further advance this field. As we look to the future, embracing these partnerships will be key to overcoming challenges and achieving breakthroughs in clinical research.

Thorough QT (TQT) studies are pivotal in clinical research, focusing on the cardiac safety of new medications by examining their effects on the QT interval. Understanding the significance of TQT is essential for clinical research leaders, as these investigations not only safeguard patient health but also ensure that new therapies meet regulatory standards. The insights gained from TQT studies inform drug development processes, helping to identify potential risks associated with new chemical entities (NCEs) and guiding the trajectory of clinical trials.
The article highlights several key points regarding TQT studies, including:
With a significant percentage of TQT evaluations still influencing regulatory decisions, it is clear that these studies remain integral to understanding the pharmacological impacts of medications. Furthermore, the historical context provided illustrates the progression of TQT studies from their inception to modern practices, emphasizing the need for continuous adaptation and innovation in methodologies.
In light of these findings, it is crucial for clinical research leaders to prioritize TQT investigations in their development strategies. By embracing the significance of TQT studies, stakeholders can enhance the safety and efficacy of new therapies, ultimately contributing to better patient outcomes. As the landscape of drug development continues to evolve, staying informed about TQT guidelines and methodologies will be essential for navigating the complexities of clinical research and ensuring that advancements in healthcare are both safe and effective.
What are Thorough QT (TQT) studies?
TQT studies are specialized clinical trials that evaluate the effects of new medications on the QT interval of the heart's electrical cycle, specifically the QTc interval, to identify any proarrhythmic hazards associated with new chemical entities (NCEs).
What is the primary objective of a TQT study?
The primary objective is to determine whether the medication extends the QTc interval by 10 milliseconds or more, a threshold that raises regulatory concerns.
How is a TQT study typically designed?
A TQT study typically employs a randomized, double-blind, placebo-controlled design, where participants receive either the investigational medication or a placebo, with their heart rhythms monitored through electrocardiograms (ECGs).
What percentage of FDA data reports are standalone TQT assessments?
Standalone TQT assessments account for approximately 50% of all data reports reviewed by the FDA.
Can you provide an example of a medication used in TQT studies?
Moxifloxacin has frequently served as a positive control in TQT investigations, demonstrating a peak effect of around 14 milliseconds.
Why are TQT studies important in medication development?
TQT studies enhance the understanding of pharmacological effects on the QT/QTc interval and provide valuable pharmacokinetic information, contributing to safer therapeutic options for patients.
When should TQT assessments be conducted in the medication development process?
Industry specialists recommend conducting TQT assessments early in the development process to mitigate risks associated with the ongoing progress of a compound.
What costs are associated with conducting TQT evaluations?
Subsequent evaluations may be necessary if proof-of-concept efficacy data is required, and the costs involved can exceed $5 million.