


The article addresses the essential practices for ensuring patient safety in accordance with COFEPRIS regulations. It emphasizes the importance of:
These practices are underpinned by:
Together, these elements are vital for protecting participants and enhancing the credibility of clinical research in Mexico.
In the intricate landscape of clinical research, understanding the regulatory framework established by COFEPRIS is paramount for ensuring patient safety and the integrity of trials. As Mexico's Federal Commission for Protection against Sanitary Risks, COFEPRIS sets forth essential guidelines that govern everything from the approval processes to post-market surveillance of medical devices. Recent advancements aimed at enhancing transparency and compliance underscore the necessity for organizations to navigate these regulations meticulously. This diligence is crucial not only to safeguard participants but also to bolster the credibility of their research. This article delves into the critical aspects of COFEPRIS regulations, emphasizing the importance of:
Thereby providing a comprehensive overview for stakeholders involved in clinical trials.
COFEPRIS, the Federal Commission for Protection against Sanitary Risks in Mexico, plays a crucial role in overseeing research involving human participants. A comprehensive understanding of COFEPRIS regulations is essential for any organization conducting research in Mexico, as these guidelines encompass the approval process, ethical considerations, and post-market surveillance requirements. Adhering to these regulations not only enhances patient safety under COFEPRIS regulations but also mitigates risks associated with adverse events, thereby safeguarding patient welfare and improving the credibility of research outcomes. This credibility fosters confidence among stakeholders and participants, which is vital for the success of research studies.
bioaccess provides extensive management services for research studies that include feasibility assessments, site selection, adherence reviews, study setup, import permits, project management, and reporting. These capabilities are designed to ensure that organizations can navigate the complexities of COFEPRIS regulations effectively. Recent updates from COFEPRIS, including the launch of Digipris, aim to improve transparency and regulatory efficiency in Mexico's clinical trials market. Such advancements underscore the significance of following regulations, as organizations that prioritize adherence to COFEPRIS guidelines can avoid substantial financial losses and reputational harm linked to non-adherence.
For instance, a case study illustrates how investing in regulatory protocols can be viewed as a cost-saving strategy, contrasting the initial expenses with the potential significant costs associated with data breaches or audits. Furthermore, it is crucial for the leader of the health organization to oversee studies involving minors, ensuring that ethical factors are addressed and hazards are assessed appropriately.
In 2025, the impact of COFEPRIS regulations on patient safety under COFEPRIS regulations remains substantial, with expert viewpoints emphasizing that strict adherence is vital for protecting participants in research trials. As noted by PPD (Thermo Fisher Scientific), adherence to these regulations is critical for maintaining the integrity of clinical research. Organizations are encouraged to regularly review COFEPRIS updates to stay informed about changes that may affect their studies, thereby ensuring patient safety under COFEPRIS regulations and upholding the highest standards of ethical conduct in their research endeavors.
Moreover, bioaccess is committed to guaranteeing information security and client trust through robust data protection measures and grievance procedures, addressing any issues with adherence and transparency.

To establish structured compliance processes, organizations must develop a robust compliance framework encompassing several critical steps:
A case study named 'Ensuring Participant Protection and Ethical Adherence' demonstrates how COFEPRIS guarantees participant protection and ethical adherence in clinical studies, highlighting the significance of patient safety under COFEPRIS regulations and organized adherence procedures. By following these steps and recognizing typical challenges, such as poor documentation or lack of training, organizations can foster a culture of adherence that emphasizes patient well-being and enhances the integrity of their research initiatives.
As the Bioaccess Content Team indicates, "bioaccess® provides customized solutions for Medtech startups, including study management services, approval processes, participant recruitment, compliance evaluations, and project oversight, ensuring a smooth transition from pilot study to commercialization.

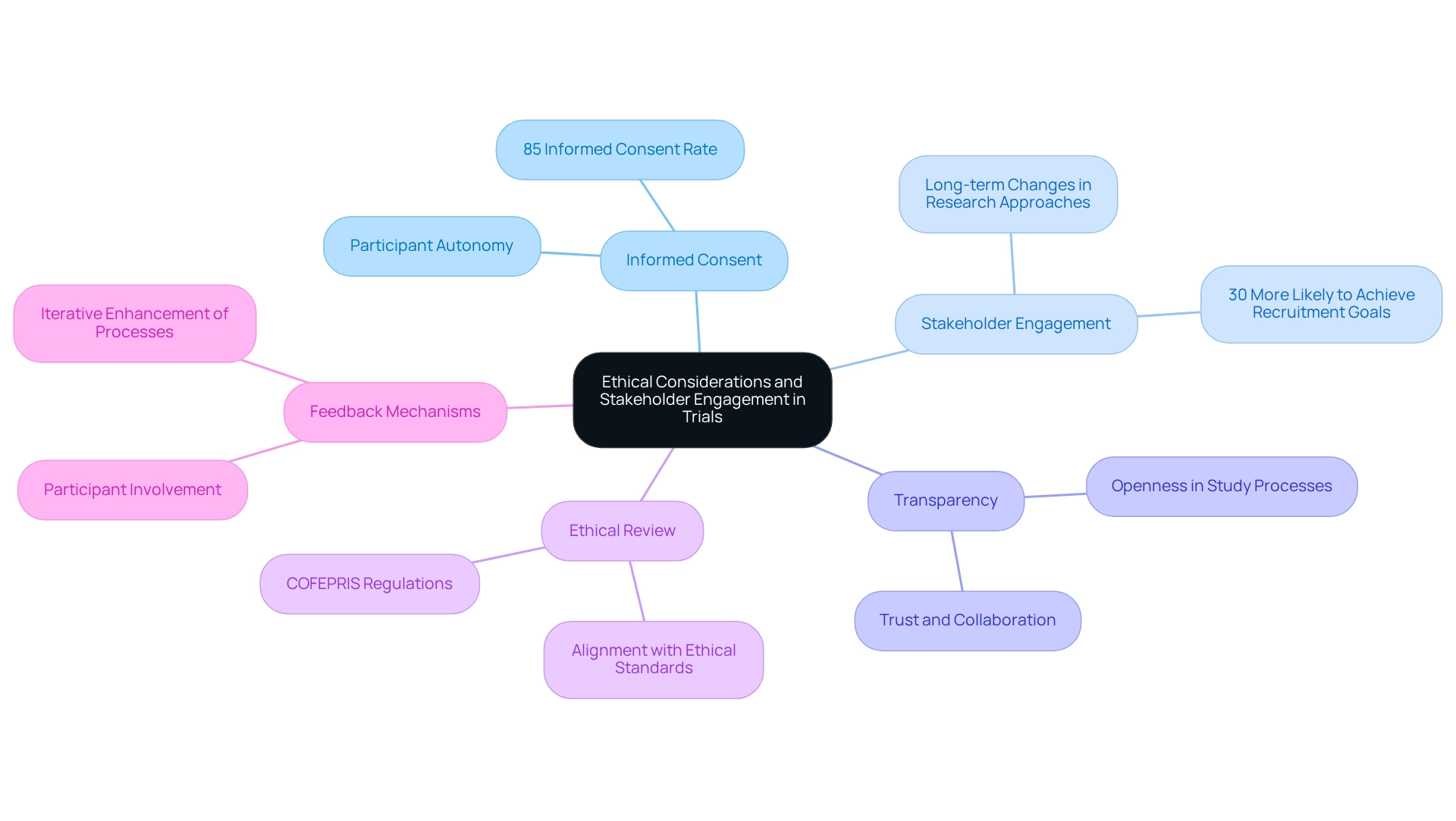
Integrating ethical factors into research studies is crucial for guaranteeing participant safety and confidence. Key practices include:
By emphasizing ethics and stakeholder involvement, organizations can bolster participant confidence and elevate the overall standard of research, ultimately resulting in more favorable outcomes. As noted by the ESAG, addressing physician concerns regarding protocol-indicated standing orders can clarify ethical considerations in clinical trials, further emphasizing the importance of stakeholder engagement.
To ensure continuous patient safety under COFEPRIS regulations through post-market surveillance, organizations must adopt essential practices.
By implementing these practices, organizations can significantly enhance patient safety under COFEPRIS regulations, ensure compliance with post-market requirements, and ultimately foster trust and innovation in the medical device sector. However, it is important to recognize the limitations of post-market surveillance, including being resource-intensive, data challenges, reporting bias, regulatory complexity, and response delays. Addressing these limitations will better prepare organizations for the challenges they may face in implementing these practices.

Understanding and adhering to the regulatory framework established by COFEPRIS is crucial for the success of clinical trials in Mexico. The significance of structured compliance processes, ethical considerations, and continuous patient safety measures cannot be overstated. By implementing a robust compliance framework, organizations can effectively navigate the complexities of COFEPRIS regulations, ensuring that all trial activities prioritize participant welfare and uphold ethical standards.
Moreover, incorporating stakeholder engagement and transparent practices into clinical research enhances trust and collaboration among participants and stakeholders alike. The emphasis on informed consent and active communication reflects a commitment to ethical conduct and participant autonomy, which are fundamental to the integrity of clinical trials.
Continuous patient safety through post-market surveillance is essential for maintaining the efficacy and safety of medical devices long after approval. By establishing comprehensive monitoring systems and encouraging open reporting mechanisms, organizations can proactively address safety concerns and foster a culture of transparency and accountability.
Navigating COFEPRIS regulations with diligence not only safeguards participants but also strengthens the credibility and integrity of clinical research. As organizations embrace these practices, they contribute to a safer and more trustworthy clinical trial environment, ultimately benefiting all stakeholders involved.
What is COFEPRIS and what role does it play in research involving human participants in Mexico?
COFEPRIS, the Federal Commission for Protection against Sanitary Risks in Mexico, oversees research involving human participants. It establishes regulations that include the approval process, ethical considerations, and post-market surveillance requirements.
Why is understanding COFEPRIS regulations important for organizations conducting research in Mexico?
A comprehensive understanding of COFEPRIS regulations is essential for organizations to enhance patient safety, mitigate risks associated with adverse events, and improve the credibility of research outcomes.
How do COFEPRIS regulations impact patient welfare and research credibility?
Adhering to COFEPRIS regulations safeguards patient welfare and enhances the credibility of research outcomes, fostering confidence among stakeholders and participants, which is vital for the success of research studies.
What services does bioaccess provide to help organizations navigate COFEPRIS regulations?
Bioaccess offers management services for research studies, including feasibility assessments, site selection, adherence reviews, study setup, import permits, project management, and reporting.
What recent updates have been made by COFEPRIS to improve regulatory efficiency?
Recent updates include the launch of Digipris, which aims to enhance transparency and regulatory efficiency in Mexico's clinical trials market.
What are the potential consequences of not adhering to COFEPRIS regulations?
Organizations that do not prioritize adherence to COFEPRIS guidelines may face substantial financial losses and reputational harm.
How can investing in regulatory protocols be viewed as a cost-saving strategy?
Investing in regulatory protocols can prevent significant costs associated with data breaches or audits, making it a cost-saving strategy when compared to initial expenses.
What specific responsibilities do leaders of health organizations have regarding studies involving minors?
Leaders must oversee studies involving minors to ensure that ethical factors are addressed and hazards are properly assessed.
How significant is the impact of COFEPRIS regulations on patient safety as of 2025?
The impact remains substantial, with expert viewpoints emphasizing that strict adherence to COFEPRIS regulations is vital for protecting participants in research trials.
What measures does bioaccess take to ensure information security and client trust?
Bioaccess guarantees information security and client trust through robust data protection measures and grievance procedures to address issues with adherence and transparency.