


This article delineates five essential steps for the implementation of effective Corrective Action and Preventive Action (CAPA) processes in clinical research. These steps include:
Each of these steps is crucial for enhancing quality management and ensuring regulatory compliance. They empower organizations to effectively identify and address nonconformities while fostering a culture of continuous improvement.
In the intricate realm of clinical research, the significance of effective corrective action and preventive action (CAPA) is paramount. Organizations continually grapple with the dual challenge of addressing existing nonconformities while also anticipating potential issues that could threaten patient safety and research integrity. This article explores five crucial steps for establishing a robust CAPA system, providing insights into how organizations can bolster compliance, promote continuous improvement, and ultimately protect their research outcomes.
What strategies can organizations implement to ensure that their CAPA processes are not merely reactive but instead proactive in averting future challenges?
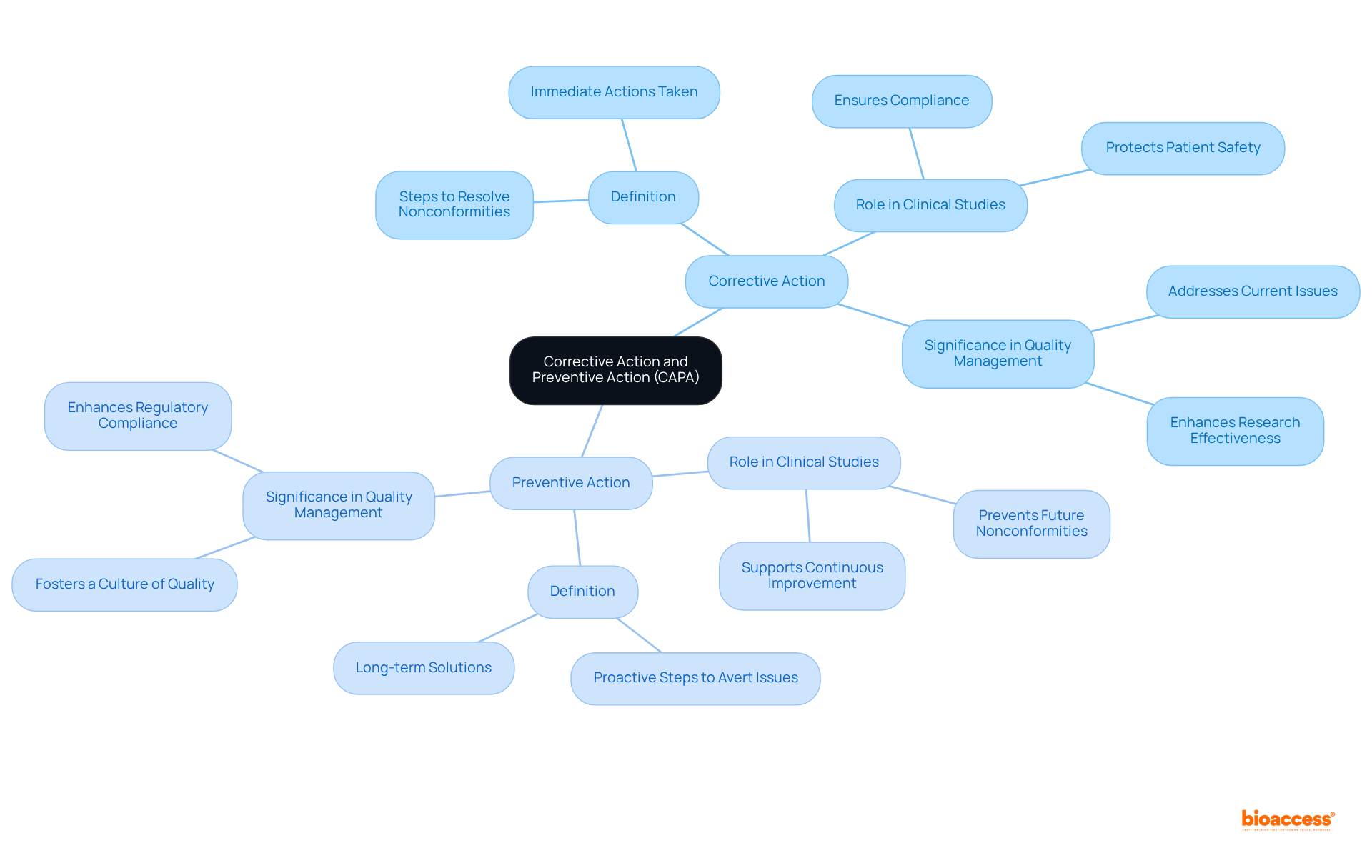
The measures implemented to eliminate the root causes of existing nonconformities or undesirable situations are part of the corrective action and preventive action CAPA, aimed at preventing their recurrence. In contrast, the process of corrective action and preventive action CAPA involves proactive steps taken to eliminate the causes of potential nonconformities, aiming to avert their occurrence altogether. In the realm of clinical studies, the corrective action and preventive action CAPA process is vital for ensuring adherence to regulatory standards and safeguarding the integrity of study outcomes. Notably, the turnaround time for corrective action and preventive action CAPA has improved from 120 days to 90 days, highlighting the efficiency of a well-structured system. Effective implementation of corrective action and preventive action CAPA not only protects patient safety but also enhances the overall effectiveness of research initiatives.
As Tom Rynkiewicz emphasizes, 'The chance to genuinely enhance their product is merely 10% of its potential,' highlighting the critical importance of a robust corrective action and preventive action CAPA system. By clearly defining these actions and referencing successful case studies, organizations can better understand their roles in quality management and risk mitigation strategies, ultimately fostering a culture of continuous improvement and regulatory compliance.
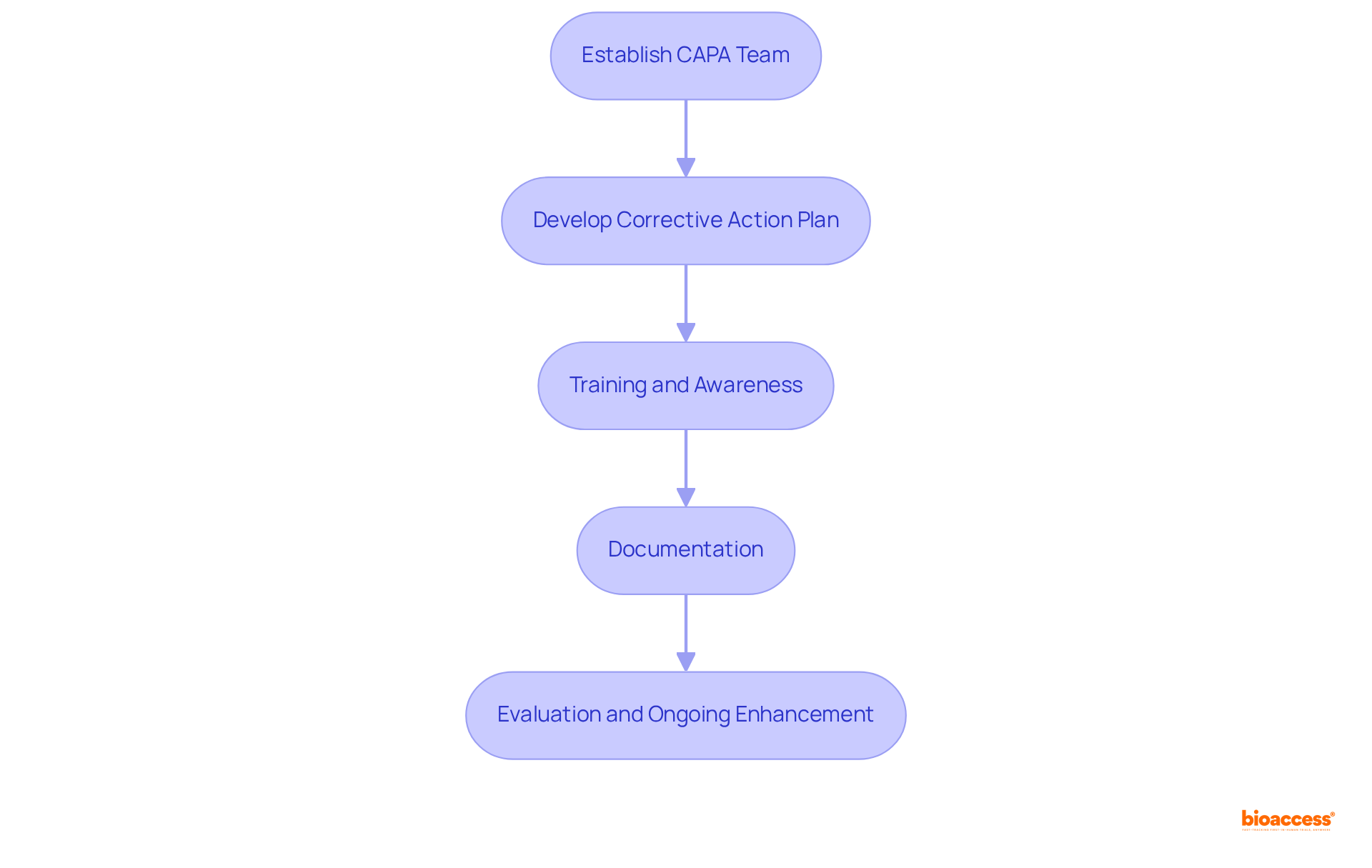
To implement effective CAPA processes in clinical research, organizations should follow these steps:
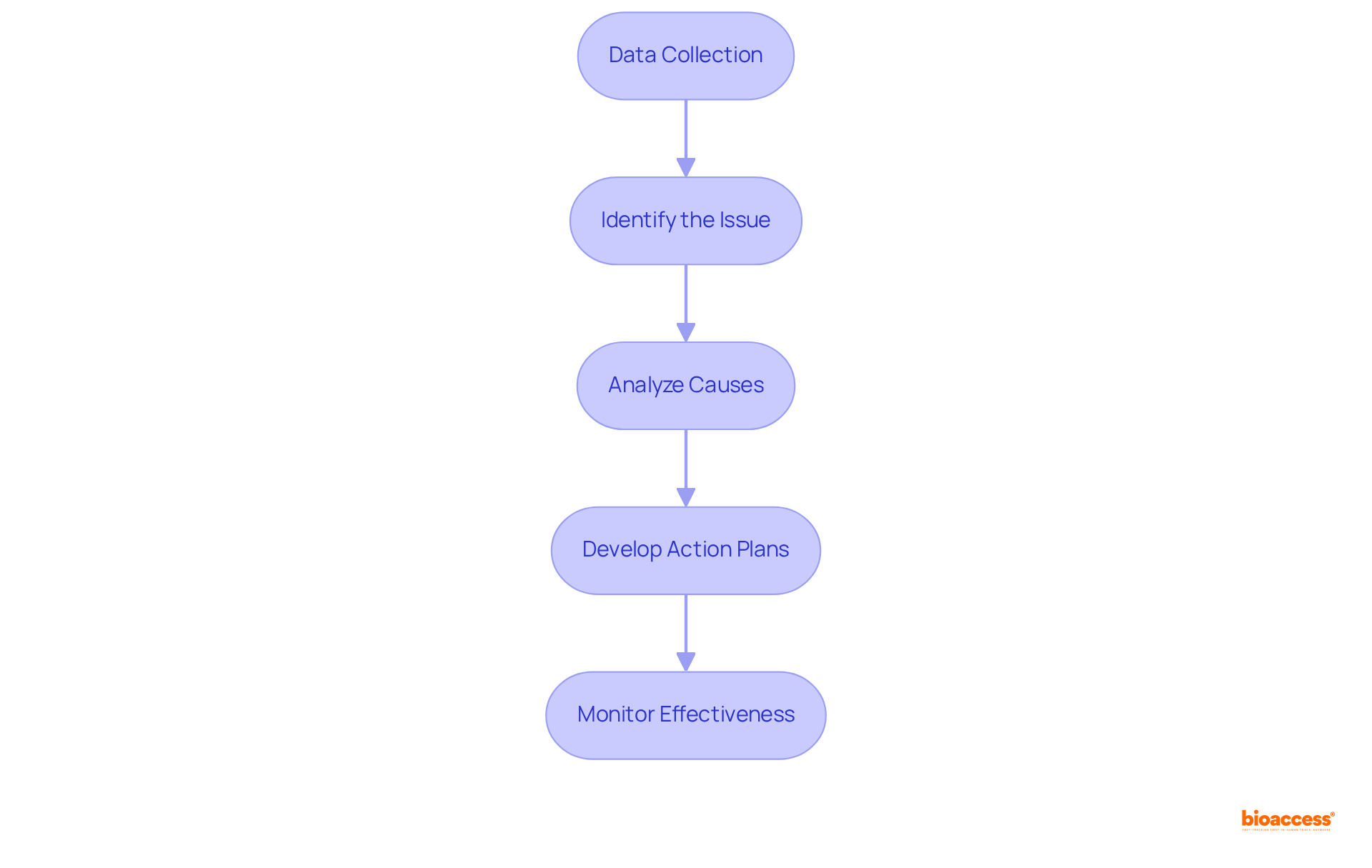
Utilizing Root Cause Analysis (RCA) is vital for the success of corrective action and preventive action capa initiatives in clinical research. To effectively conduct RCA, follow these key steps:
Data Collection: Collect comprehensive data related to the nonconformity, including incident reports, audit findings, and stakeholder feedback. Effective data gathering is essential; approximately 29% of companies indicate that several departments are engaged in the RCA procedure, with 41% involving three departments. This underscores the necessity for a collaborative approach.
Identify the Issue: Clearly outline the issue and its effect on the study. It is imperative that all team members understand the issue, as a well-defined problem is essential for effective resolution.
Analyze Causes: Employ techniques such as the 5 Whys or Fishbone Diagram to investigate potential causes of the problem. Engaging cross-functional team members in this analysis can provide diverse perspectives, which is critical, given that common causes of nonconformities in clinical trials often stem from communication errors and procedural inconsistencies.
Develop Action Plans: Create targeted action plans based on the identified root causes. Each plan should include specific corrective action and preventive action capa tailored to effectively address the underlying issues. This step is crucial, as the average cost of a re-run in clinical trials can reach up to $4,870, with the annual cost of re-runs totaling $233,000, emphasizing the financial impact of unresolved problems.
Monitor Effectiveness: After implementing the action plans, continuously monitor their effectiveness to ensure that the issues have been resolved and do not recur. This ongoing evaluation is essential for maintaining quality and compliance in clinical research, fostering a culture of continuous improvement and accountability. As Kevin Lee, Sales Solutions Engineer at SafetyChain Software, states, "Only by understanding the root cause of the issue can you stop it from recurring.
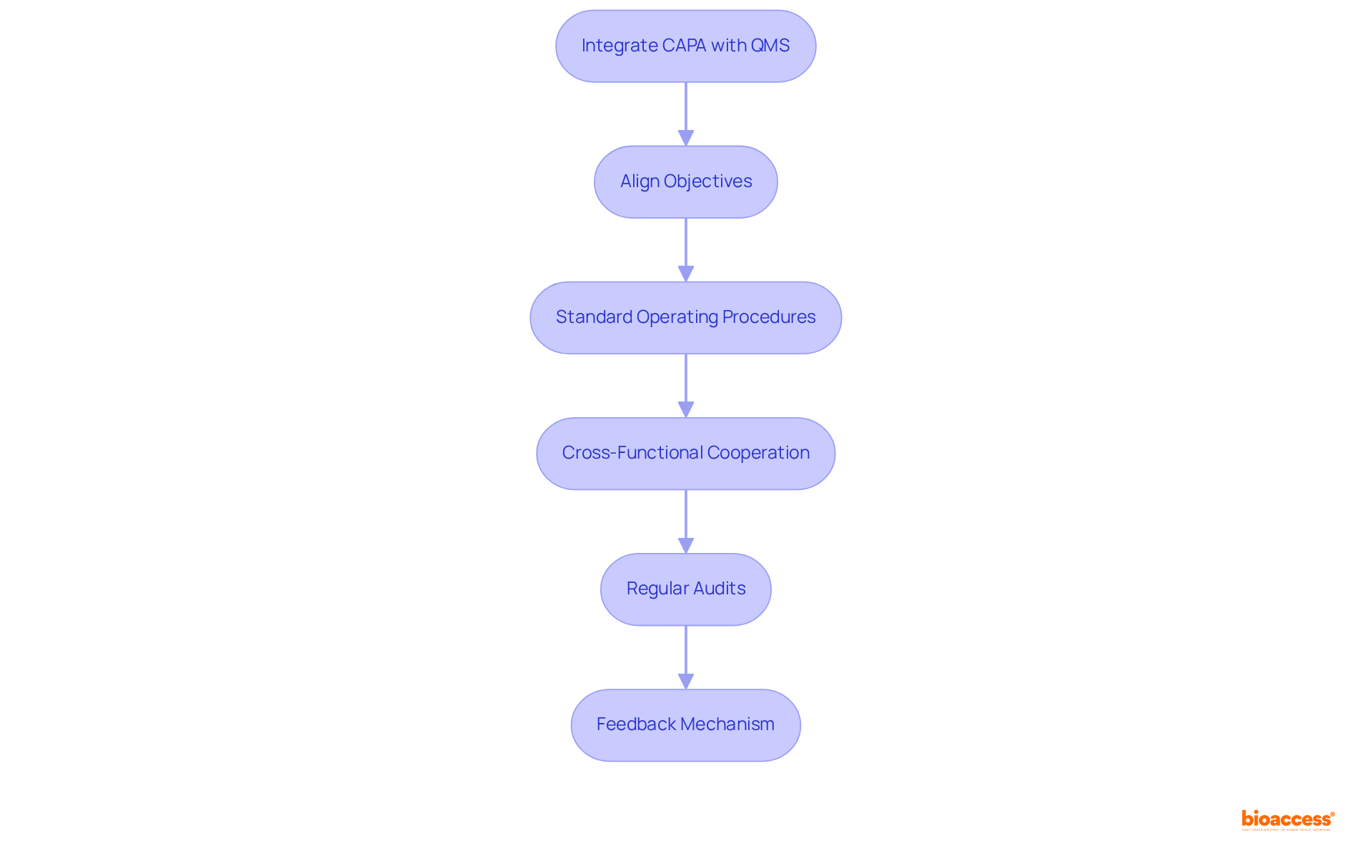
Integrating quality management systems with corrective action and preventive action capa is essential for maximizing the effectiveness of both processes. To achieve this integration, consider the following key steps:
Align Objectives: Ensure that corrective and preventive action objectives are closely aligned with the overarching goals of the QMS. This alignment fosters a cohesive approach to quality assurance, enhancing overall effectiveness. As Thirumalai Arunagiri observes, the primary objective of corrective action and preventive action capa is to identify weaknesses and implement measures to prevent their recurrence, which is essential for upholding high standards in the pharmaceutical sector.
Standard Operating Procedures (SOPs): Create detailed SOPs that incorporate corrective and preventive action processes within the QMS framework. This ensures that all personnel are aware of their roles and responsibilities, promoting accountability and consistency. The FDA's Code of Federal Regulations emphasizes the importance of structured procedures in maintaining compliance.
Cross-Functional Cooperation: Promote teamwork among divisions engaged in management and clinical research. This cross-disciplinary method guarantees that corrective and preventive actions are guided by a comprehensive understanding of issues, resulting in more effective solutions. A case study on the application of corrective action and preventive action capa in pharmaceutical production demonstrates how cross-departmental efforts can improve quality results.
Regular Audits: Conduct routine evaluations of both corrective actions and quality management systems. These audits help identify areas for improvement and ensure compliance with regulatory standards, such as those outlined in the FDA's Code of Federal Regulations. Regular audits are essential for fostering a culture of continuous improvement, which is supported by the corrective action and preventive action capa as highlighted by ISO 9001:2000 principles.
Feedback Mechanism: Establish a robust feedback system that facilitates continuous enhancement of both corrective actions and QMS processes. This allows organizations to learn from past experiences and integrate those lessons into future practices, fostering a culture of ongoing enhancement. As highlighted by Kanaka P. Kannaiah, the implementation of corrective action and preventive action capa not only addresses current performance issues but also enhances the organization's capacity to foresee and reduce potential risks.
By adhering to these steps, organizations can successfully merge their QMS with corrective action and preventive action capa, ultimately resulting in enhanced results in clinical trials and beyond. Incorporating these practices can result in enrollment that is 50% faster than traditional markets, demonstrating the tangible benefits of a well-integrated quality management approach.
In conclusion, prioritizing robust CAPA processes is not merely a regulatory obligation; it represents a strategic advantage that can significantly influence the success of clinical trials. Organizations are strongly encouraged to adopt these best practices, fostering a proactive culture that values quality and compliance. By doing so, they can effectively mitigate risks while simultaneously driving innovation and excellence in research initiatives. Ultimately, this approach benefits not only patients but also enhances the broader healthcare landscape.
What is the purpose of Corrective Action and Preventive Action (CAPA)?
CAPA aims to eliminate the root causes of existing nonconformities or undesirable situations to prevent their recurrence and involves proactive steps to eliminate the causes of potential nonconformities, thereby averting their occurrence altogether.
Why is CAPA important in clinical studies?
The CAPA process is vital in clinical studies for ensuring adherence to regulatory standards and safeguarding the integrity of study outcomes.
How has the turnaround time for CAPA improved?
The turnaround time for CAPA has improved from 120 days to 90 days, indicating the efficiency of a well-structured system.
What benefits does effective CAPA implementation provide?
Effective CAPA implementation protects patient safety and enhances the overall effectiveness of research initiatives.
What does Tom Rynkiewicz emphasize regarding CAPA?
Tom Rynkiewicz emphasizes that the chance to genuinely enhance a product is merely 10% of its potential, highlighting the critical importance of a robust CAPA system.
How can organizations benefit from clearly defining CAPA actions?
By clearly defining CAPA actions and referencing successful case studies, organizations can better understand their roles in quality management and risk mitigation strategies, fostering a culture of continuous improvement and regulatory compliance.