


Developing a medical device is a journey that is both crucial and complex. It involves a series of meticulously defined stages designed to ensure safety, efficacy, and market readiness. This guide outlines the five essential steps of the product development process, highlighting how each phase - from conceptualization to post-market surveillance - plays a vital role in creating innovative solutions that address real-world healthcare needs.
With the landscape rapidly evolving and regulatory requirements becoming increasingly stringent, developers face the challenge of navigating these complexities. How can they ensure their products not only comply but also stand out in a competitive market?
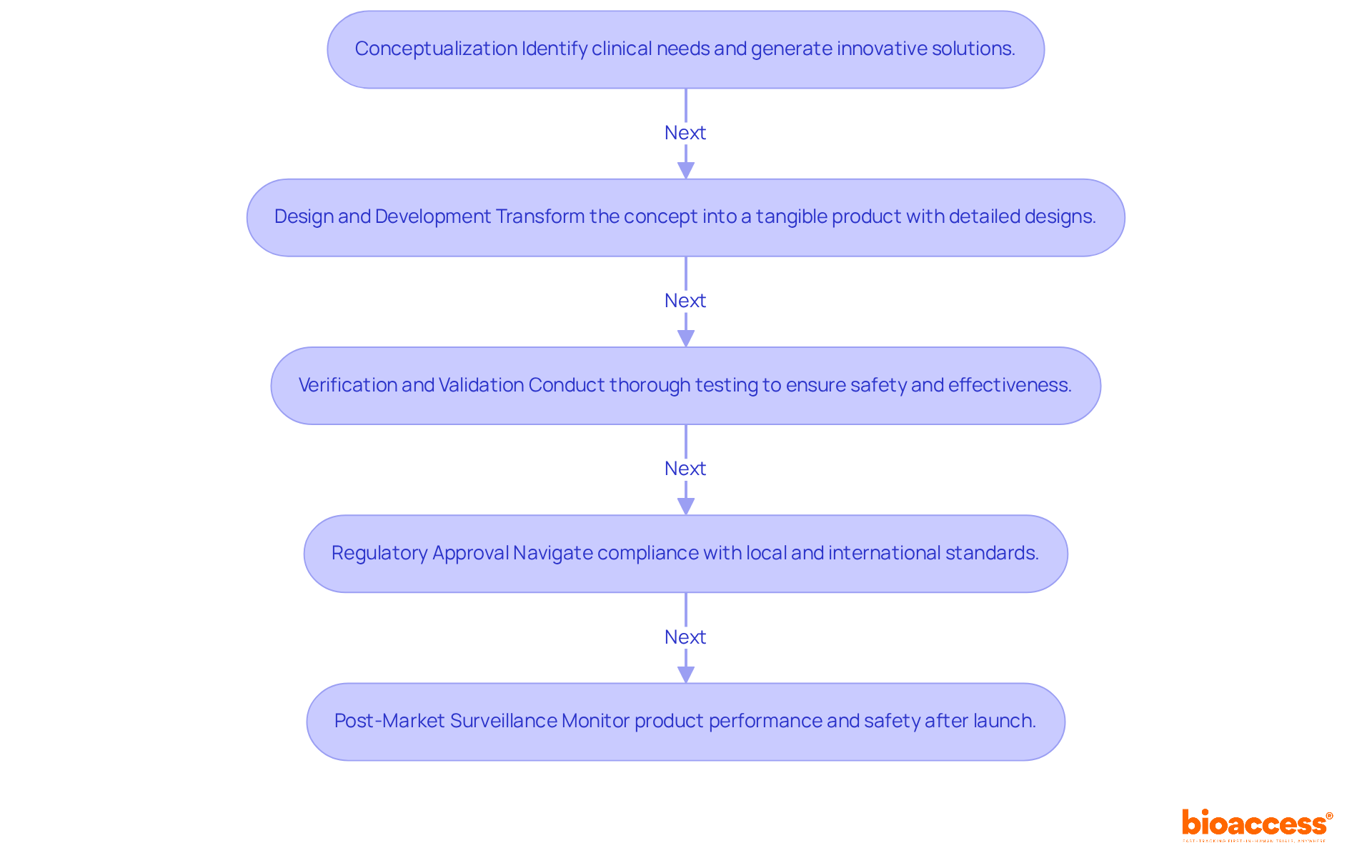
The product development process for medical devices is a multi-faceted journey that encompasses several critical stages, each essential for ensuring successful design, manufacturing, and market entry.
Conceptualization: This foundational phase focuses on identifying clinical needs and generating innovative solutions. Documenting ideas and assessing their feasibility is crucial, as it sets the groundwork for subsequent development. Recent trends emphasize the significance of a human-centered approach, which improves usability and aligns with user expectations.
Design and Development: During this stage, the initial concept evolves into a tangible product. Detailed creation tasks, including engineering specifications and prototypes, are conducted. Sophisticated creation tools and methods, such as Design for 'X' activities, are utilized to ensure precision and manufacturability. This stage generally requires several months, depending on the intricacy of the apparatus.
Verification and Validation: This essential phase entails thorough testing to ensure that the device meets its specifications and user requirements. Validation tests are conducted under real-life conditions, ensuring that the product is safe and effective. Feedback from these tests is crucial for improving the design and ensuring adherence to legal standards.
Regulatory Approval: Navigating the regulatory landscape is vital for compliance with local and international standards before market entry. Most medical instruments require FDA approval or clearance, such as 510(k) clearance, which can take anywhere from a few months to several years, depending on the classification and complexity. Understanding these requirements is crucial for successful market entry.
Post-Market Surveillance: After launch, continuous monitoring of the product's performance and safety is necessary. This phase ensures that any emerging issues are addressed promptly, maintaining compliance and enhancing patient safety. Effective post-market surveillance contributes to long-term success in the market, as it allows for ongoing improvements based on user feedback and performance data.
Successful case studies illustrate the significance of thorough conceptualization and adherence to regulatory standards throughout the product development process for medical devices. As highlighted by industry specialists, explicitly outlining the aim and specifications of the offering establishes the groundwork for effective later stages of development, ultimately resulting in innovative and user-friendly medical solutions.
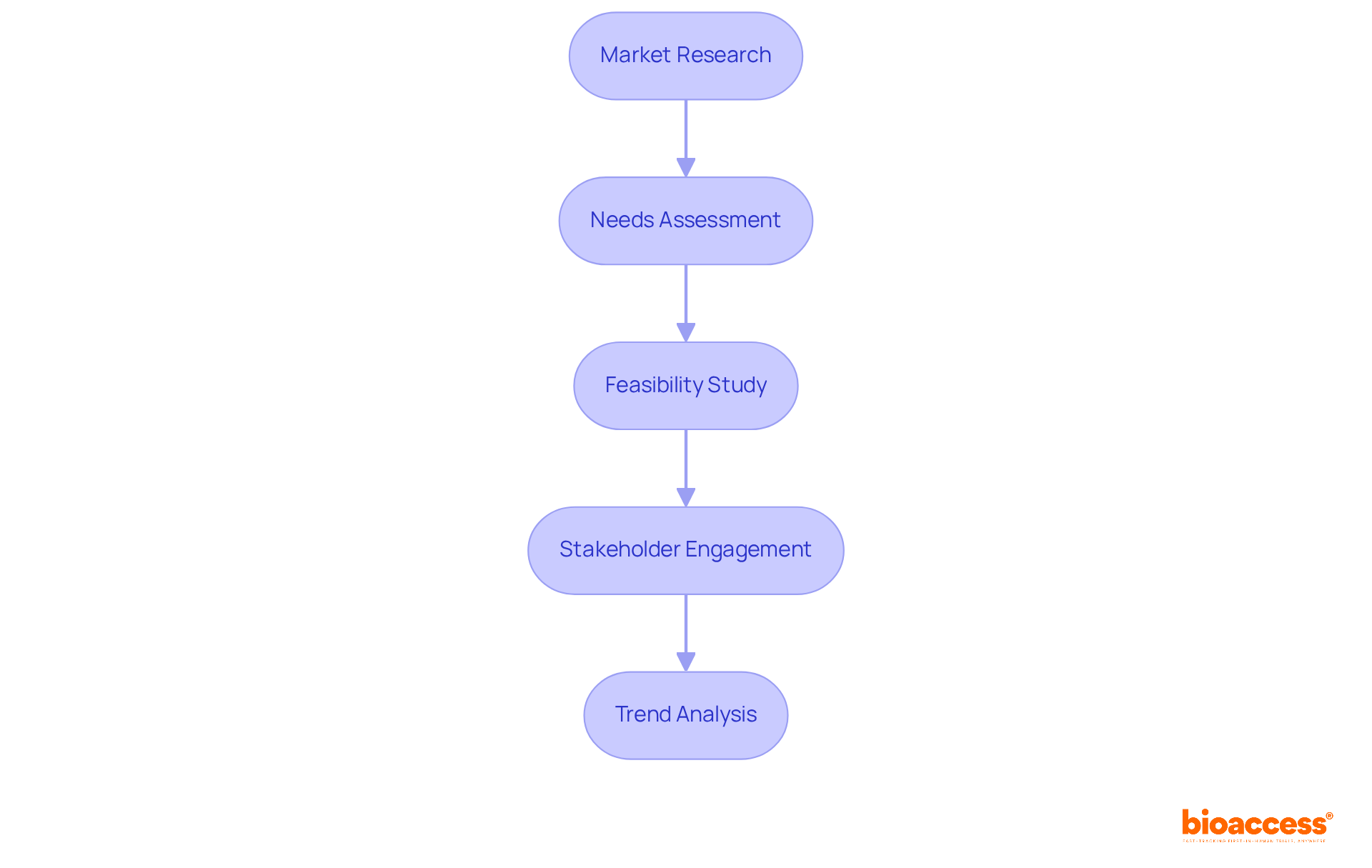
To effectively identify market needs and conduct a feasibility analysis, follow these essential steps:
Market Research: Begin by gathering comprehensive information on existing offerings, potential competitors, and target demographics. Utilize surveys, focus groups, and industry reports to gain valuable insights. Notably, the medical equipment industry is anticipated to expand substantially in 2025, driven by innovations and enhanced regulatory frameworks. This underscores the significance of thorough market analysis in a rapidly evolving environment.
Needs Assessment: Next, analyze the data to pinpoint specific needs within the market. Consider critical factors such as patient outcomes, healthcare provider requirements, and technological advancements that could influence the product development process of medical devices.
Feasibility Study: Evaluate the technical, financial, and operational aspects of the proposed device. Assess whether the project is viable in terms of cost, resources, and compliance with increasingly stringent regulatory requirements. Engaging with bioaccess® can facilitate this process, as they offer comprehensive clinical trial management services, including feasibility studies, site selection, compliance reviews, and trial setup. This ensures that your project meets all necessary standards. Additionally, bioaccess® connects startups with top-ranked clinical research sites in Latin America, Eastern Europe, and Australia, which can accelerate clinical study results and assist in raising funds.
Stakeholder Engagement: Involve key stakeholders, including healthcare professionals and potential users, to validate findings and refine the concept based on their feedback. Emphasizing the importance of real-world evidence and risk management for market access and reimbursement is crucial in this process.
Trend Analysis: Finally, stay informed about current trends in the medical device market, such as the growing demand for personalized healthcare solutions and the integration of digital technologies. Understanding these trends will help you align your product development process medical device with market expectations and enhance its potential for success.
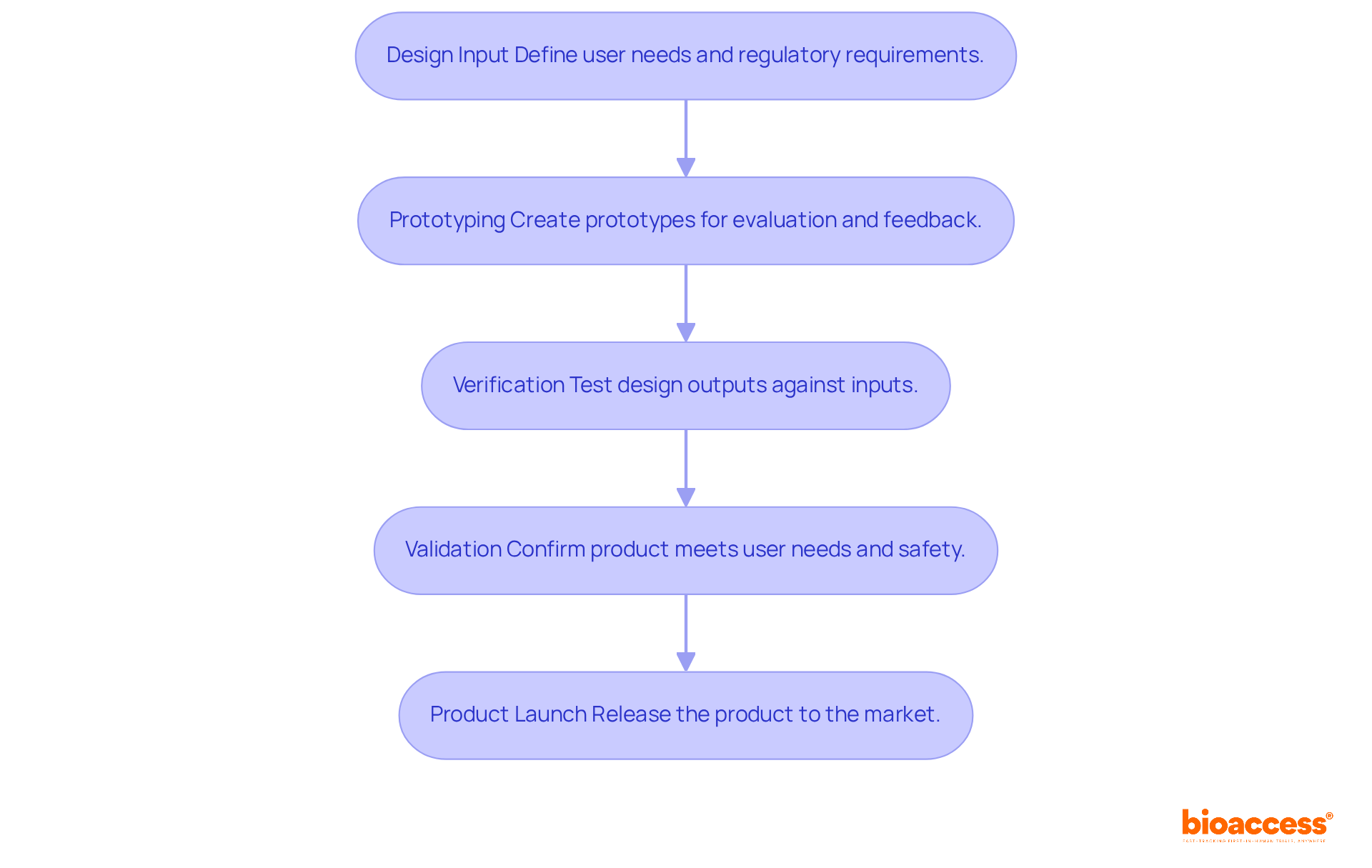
To successfully execute design and development, follow these steps:
Design Input: Clearly define user needs and regulatory requirements that the device must meet. Document these inputs meticulously to guide the development process effectively.
Prototyping: Create prototypes to visualize and evaluate the concept. This phase can include low-fidelity models for initial feedback and high-fidelity prototypes for rigorous testing. Utilize advanced techniques such as 3D printing and digital twins to enhance functionality and biocompatibility.
Verification: Implement tests to ensure that design outputs align with specified design inputs. This may involve performance testing, usability assessments, and compliance checks. Focus on achieving demonstrable reductions in both time and cost through structured sprints and on-site validation.
Validation: Validate the final outcome by confirming it meets user needs and intended uses. This frequently encompasses clinical trials or user testing to collect real-world feedback. Ensure that the product is not only effective but also safe for its intended application. Successful case studies have shown that engaging with expert teams can significantly accelerate development timelines and improve cost structures, ultimately leading to a more robust product launch.
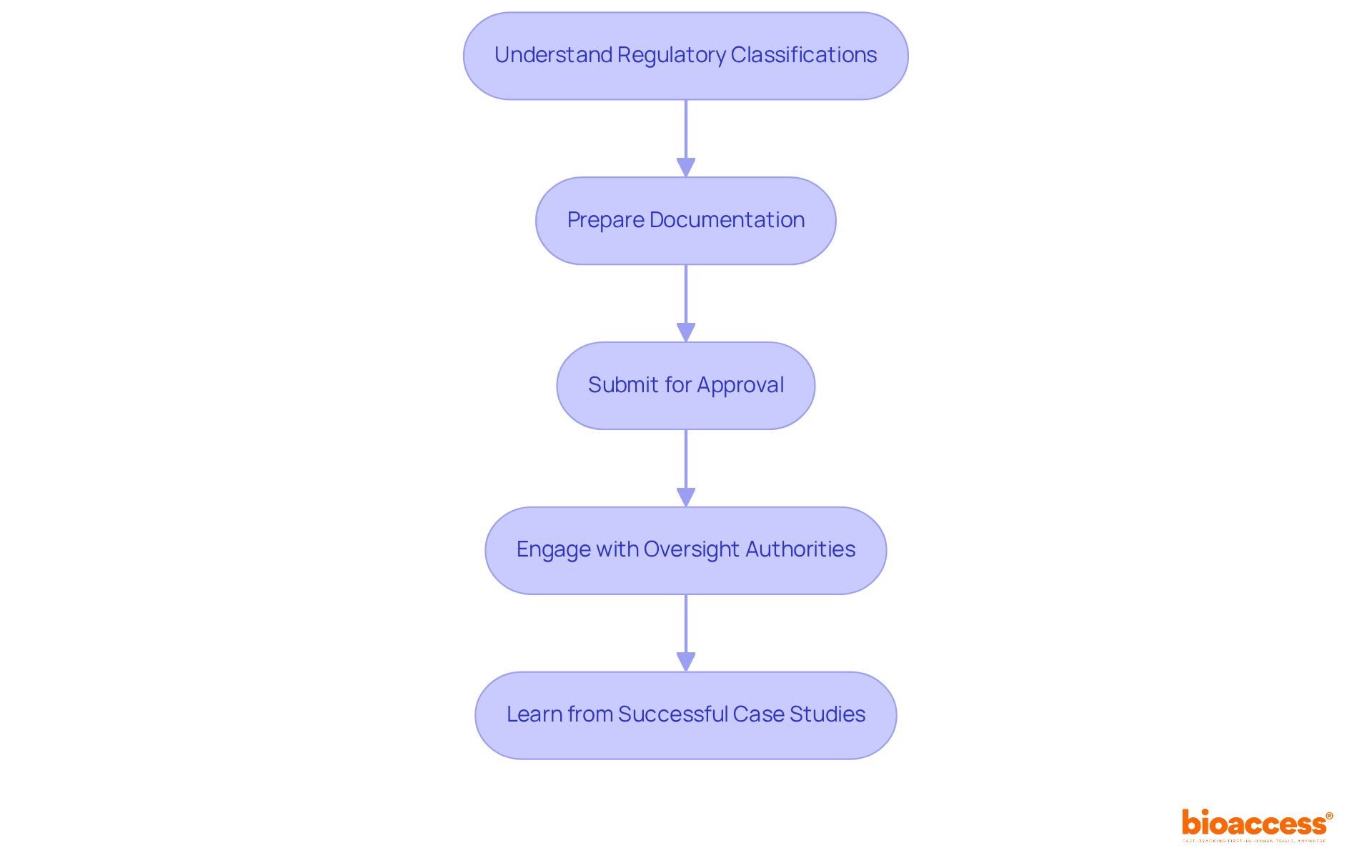
To effectively navigate regulatory approval and compliance for medical devices, follow these essential steps:
Understand Regulatory Classifications: Identify the classification of your equipment - Class I, II, or III - based on its risk level and intended use. This classification is crucial as it determines the regulatory pathway you must follow. For example, the FDA's 510(k) application process is the most common path for products that are substantially equivalent to existing items, while higher-risk products may need a more stringent Premarket Approval (PMA).
Prepare Documentation: Gather all necessary documentation, including design history files, risk management reports, and clinical data. A well-organized submission is vital; nearly 32% of FDA 510(k) submissions failed the acceptance for review check in recent years, often due to inadequate documentation.
Submit for Approval: Based on your device's classification, submit the appropriate application to the relevant governing authority, such as the FDA or EMA. The average time for a decision on 510(k) applications is approximately five months, highlighting the importance of timely and accurate submissions.
Engage with Oversight Authorities: Foster open communication with oversight agencies throughout the approval process. Be proactive in responding to inquiries and providing additional information, as 67% of 510(k) submissions received requests for additional information during the review process, which can lead to delays.
Learn from Successful Case Studies: Analyze successful regulatory submissions to understand best practices. A significant portion of 510(k) applications (85%) received a Substantially Equivalent decision, indicating effective strategies in documentation and communication that can serve as a model for your submissions.
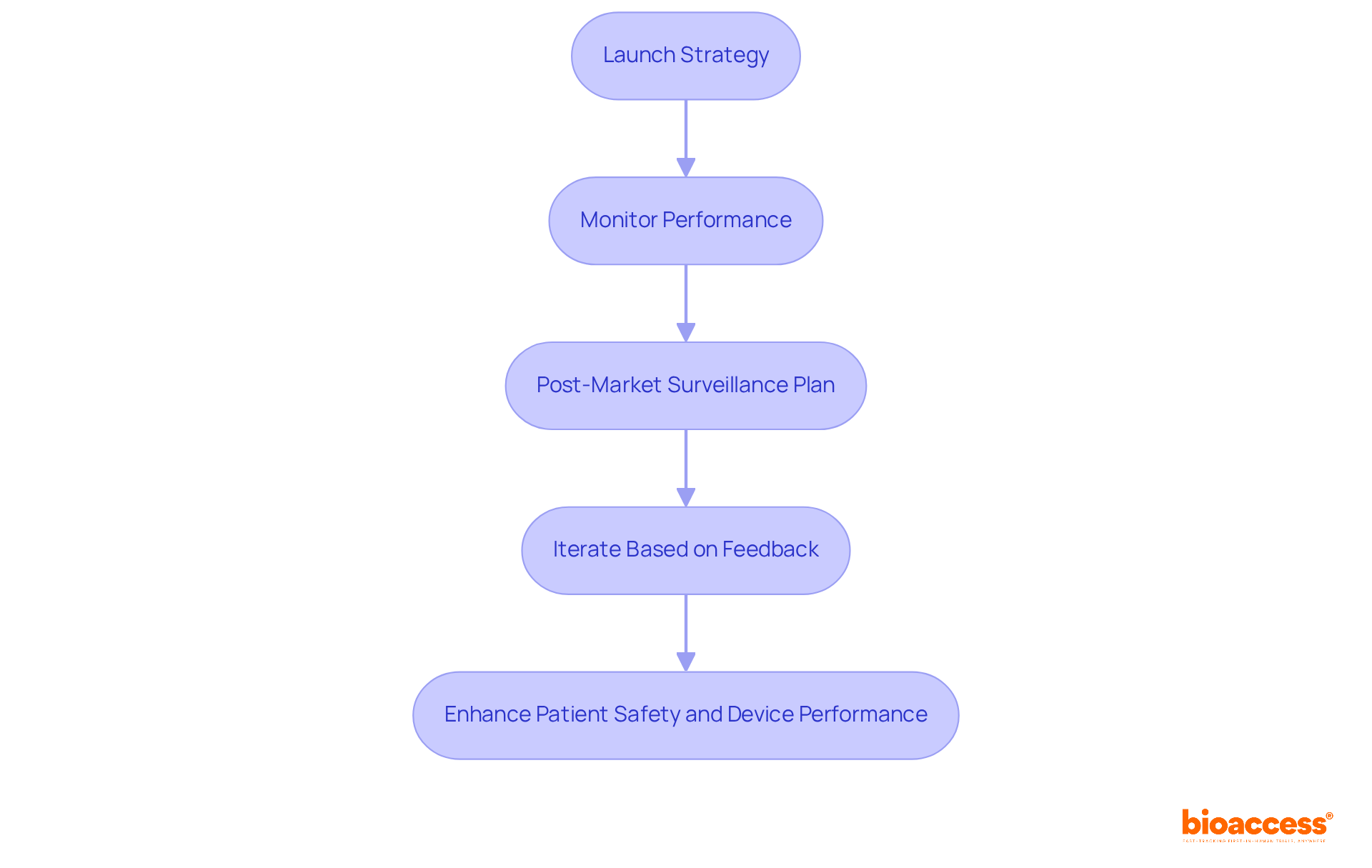
To successfully launch a product and implement effective post-market surveillance, it’s essential to follow these strategic steps:
Launch Strategy: Begin by developing a comprehensive launch plan that includes marketing strategies, distribution channels, and training for healthcare providers. This foundational step ensures that all stakeholders are well-prepared and informed about the features and benefits of the product development process medical device.
Monitor Performance: After the launch, it’s crucial to continuously track the system's performance through user feedback, clinical outcomes, and adverse event reporting. Notably, over 1.2 million medical equipment adverse event reports were not submitted to the FDA within the required timeframe, underscoring the importance of careful monitoring to swiftly identify safety issues.
The post-market surveillance plan should be part of the product development process for medical devices, establishing a robust strategy for how data will be collected and analyzed to ensure ongoing compliance and safety. This plan must align with regulatory requirements, as the FDA mandates that manufacturers submit post-market surveillance plans within 15 months of receiving an order. Effective surveillance is particularly vital for Class II and III devices, which may pose serious health risks if they fail.
Iterate Based on Feedback: Leverage insights gained from post-market surveillance to make necessary adjustments to the product or its usage guidelines. This iterative product development process medical device not only enhances safety and effectiveness but also ensures compliance with evolving regulatory standards. For example, implementing automated systems for data management can significantly boost the efficiency of post-market surveillance activities, enabling timely responses to identified issues.
By prioritizing these steps, manufacturers can enhance patient safety and device performance, ultimately fostering a culture of quality that is essential for long-term success in the Medtech industry.
The journey of developing a medical device is complex, demanding a thorough understanding of various critical stages for success. Each phase - from conceptualization to post-market surveillance - serves a unique purpose, ensuring that the final product is not only innovative but also safe and effective for users. By adopting a structured approach, manufacturers can navigate the complexities of the medical device landscape with increased confidence.
Key insights highlight the necessity of comprehensive market research, stakeholder engagement, and strict adherence to regulatory requirements. Identifying market needs and conducting feasibility analyses are foundational steps that pave the way for successful design and development. Furthermore, rigorous verification and validation processes are vital for ensuring compliance and meeting user expectations, while effective post-market surveillance is crucial for maintaining product safety and performance after launch.
Ultimately, the medical device product development process requires a steadfast commitment to quality and continuous improvement. By embracing these essential steps and prioritizing user feedback, manufacturers can enhance patient safety, drive innovation, and secure long-term success in the competitive Medtech industry. Engaging with emerging trends and maintaining a proactive approach to compliance will not only position companies for growth but also contribute to improved healthcare outcomes on a global scale.
What are the main stages of medical device product development?
The main stages of medical device product development are Conceptualization, Design and Development, Verification and Validation, Regulatory Approval, and Post-Market Surveillance.
What is the focus of the Conceptualization phase?
The Conceptualization phase focuses on identifying clinical needs and generating innovative solutions, documenting ideas, and assessing their feasibility while emphasizing a human-centered approach to improve usability.
What occurs during the Design and Development stage?
During the Design and Development stage, the initial concept evolves into a tangible product through detailed tasks such as creating engineering specifications and prototypes, utilizing sophisticated tools and methods to ensure precision and manufacturability.
Why is Verification and Validation important in medical device development?
Verification and Validation are crucial because they involve thorough testing to ensure the device meets specifications and user requirements, validating its safety and effectiveness under real-life conditions.
What is involved in the Regulatory Approval stage?
The Regulatory Approval stage involves navigating the regulatory landscape to comply with local and international standards, often requiring FDA approval or clearance, which can take several months to years depending on the device's classification and complexity.
What is Post-Market Surveillance and why is it necessary?
Post-Market Surveillance is the continuous monitoring of a product's performance and safety after launch, ensuring any emerging issues are addressed promptly, which helps maintain compliance and enhances patient safety.
How can market needs be identified for medical device development?
Market needs can be identified through market research, needs assessment, feasibility studies, stakeholder engagement, and trend analysis to gather insights on existing offerings, competitors, and specific requirements within the market.
What is the purpose of a feasibility study in medical device development?
A feasibility study evaluates the technical, financial, and operational aspects of a proposed device to determine its viability in terms of cost, resources, and compliance with regulatory requirements.
Why is stakeholder engagement important in the product development process?
Stakeholder engagement is important as it involves key stakeholders, including healthcare professionals and potential users, to validate findings and refine the concept based on their feedback, emphasizing real-world evidence and risk management.
What current trends should be considered in medical device product development?
Current trends to consider include the growing demand for personalized healthcare solutions and the integration of digital technologies, which can enhance the product's alignment with market expectations and its potential for success.