


The article delineates a five-step process for acquiring clinical trial insurance tailored specifically for radiopharmaceuticals. It underscores the necessity of comprehending various coverage types, evaluating distinct insurance requirements, and choosing reputable providers. This methodology is bolstered by comprehensive explanations of essential coverage areas, including:
Furthermore, it emphasizes the paramount significance of adhering to regulatory requirements to mitigate the risks associated with clinical research.
Navigating the intricate landscape of clinical trials necessitates not only rigorous research but also robust safeguards against potential liabilities. For sponsors and researchers engaged in radiopharmaceuticals, comprehending the process of acquiring clinical trial insurance is vital to mitigate risks associated with participant injuries, property damage, and legal claims.
However, with an array of coverage options and unique considerations, how can one ensure adequate protection while adhering to regulatory requirements?
This article examines the essential steps for securing clinical trial insurance specifically tailored for radiopharmaceuticals, empowering stakeholders to make informed decisions that protect their research endeavors.
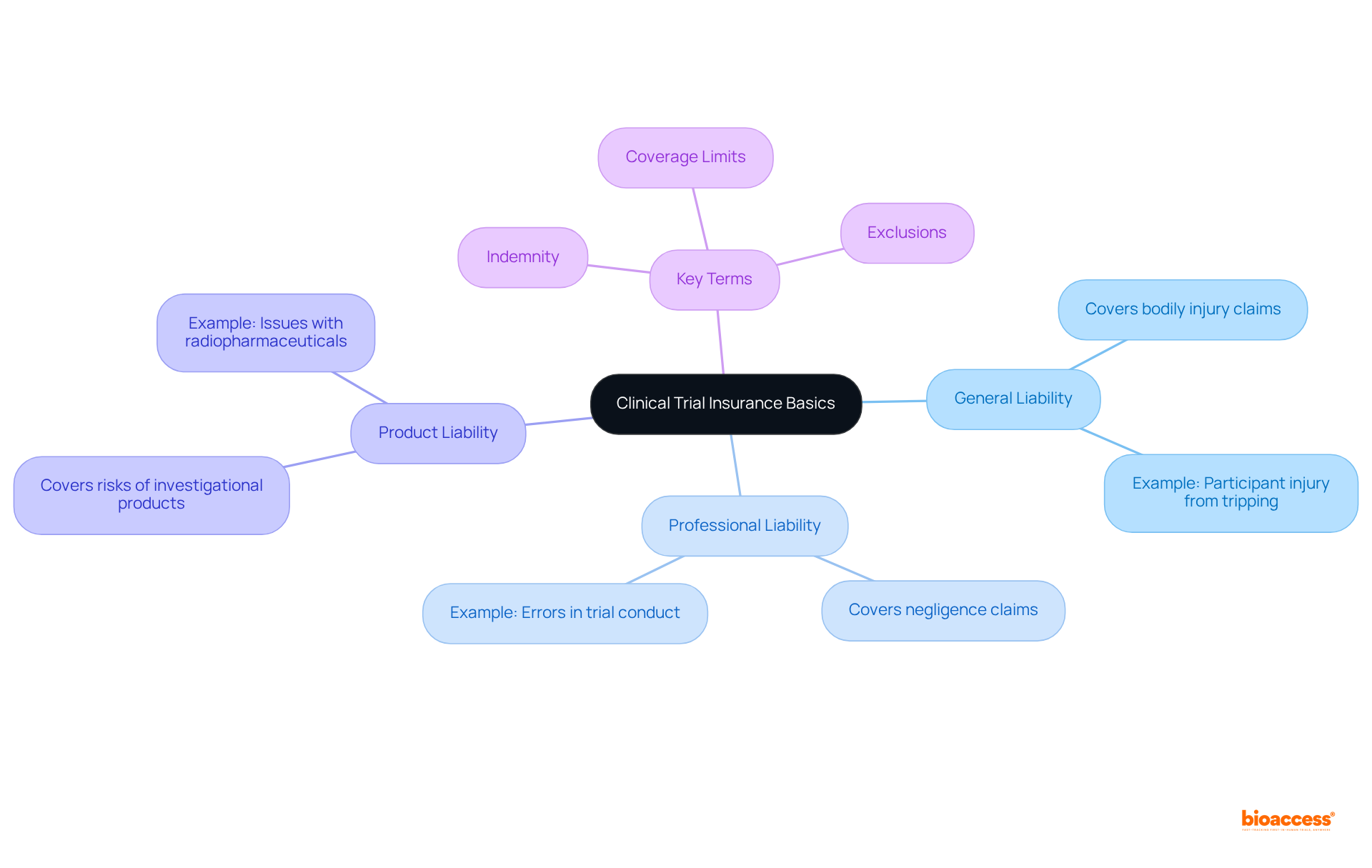
To protect against potential liabilities associated with conducting clinical studies, it is essential to buy clinical trial insurance radiopharmaceuticals for sponsors and researchers. To mitigate a range of risks, including participant injuries, property damage, and legal costs stemming from related claims, it is essential to buy clinical trial insurance radiopharmaceuticals. Understanding the various types of coverage available is essential. Key categories include:
Familiarizing yourself with terms such as 'indemnity,' 'coverage limits,' and 'exclusions' is crucial for effectively navigating the coverage landscape. Indemnity refers to the insurer's obligation to compensate for losses, while coverage limits define the maximum amount the insurer will pay for a claim. Exclusions detail specific circumstances or conditions not covered by the policy.
In 2025, the average expenses of trial coverage may vary significantly depending on the scope and nature of the study. According to industry insights, understanding these foundational elements will empower you to make informed decisions as you navigate how to buy clinical trial insurance radiopharmaceuticals. As emphasized by industry experts, possessing thorough coverage is not merely a regulatory obligation but a strategic necessity to protect against the myriad risks inherent in medical research.
Radiopharmaceuticals, which utilize radioactive substances, present distinct hazards for both participants and researchers in clinical studies. When assessing your coverage needs, it is crucial to consider several factors, such as:
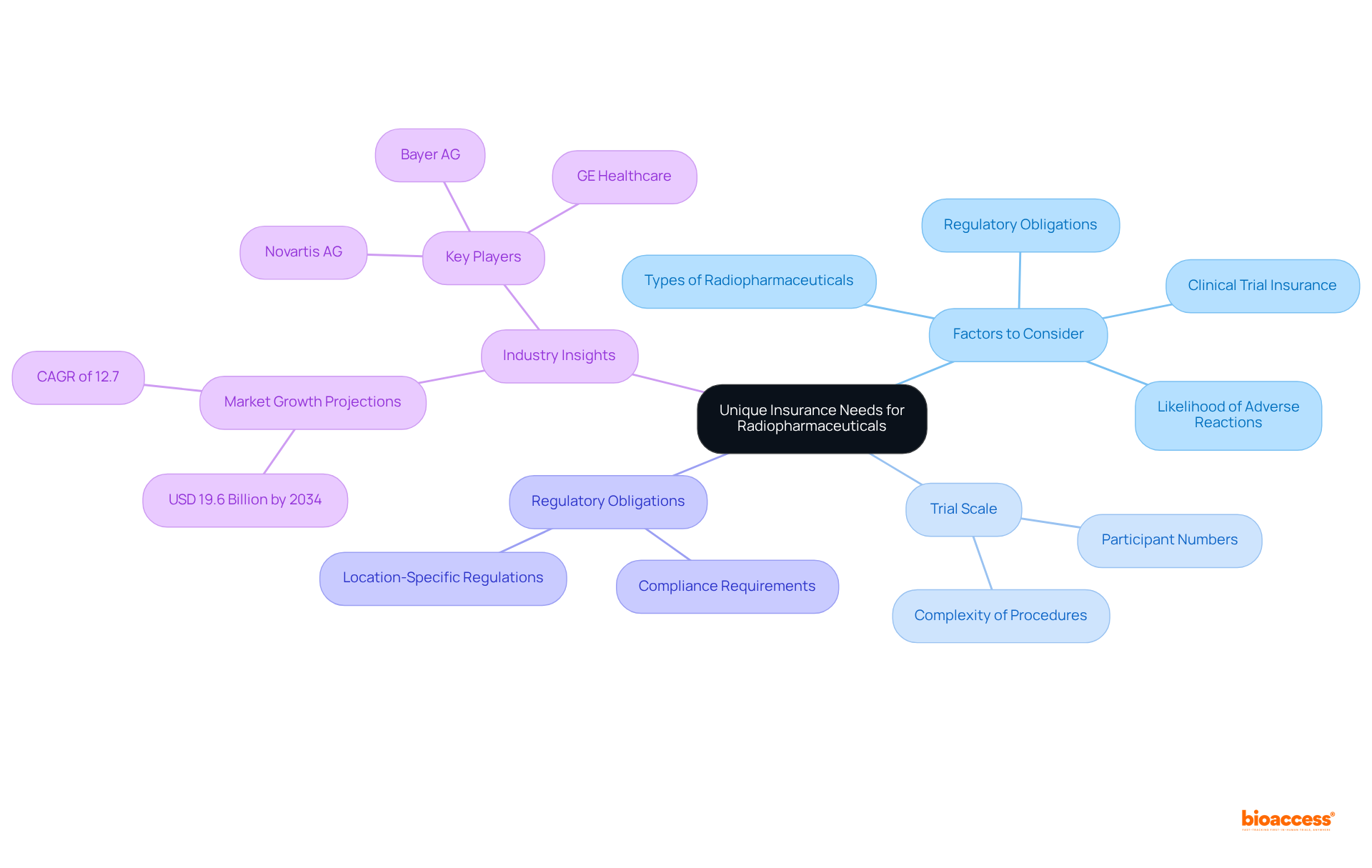
Additionally, evaluating the trial's scale—including participant numbers and the complexity of procedures—will clarify your insurance requirements. For example, the oncology segment, which leads the radiopharmaceutical market, is particularly sensitive to these risks due to the increasing incidence of cancer and the complexities associated with treatment protocols. This comprehensive assessment will assist you in determining the appropriate coverage limits and policy types necessary to buy clinical trial insurance radiopharmaceuticals and mitigate potential liabilities.
As highlighted by industry experts, grasping these unique risks is vital for ensuring robust protection in the evolving realm of radiopharmaceutical research. Engaging extensive clinical study management services, such as those offered by bioaccess, can significantly diminish these risks. Their expertise encompasses:
All essential for conducting experiments efficiently and in line with regulatory standards. This thorough understanding of coverage requirements is critical, particularly as the global radiopharmaceuticals market is anticipated to expand significantly, reaching USD 19.6 billion by 2034.
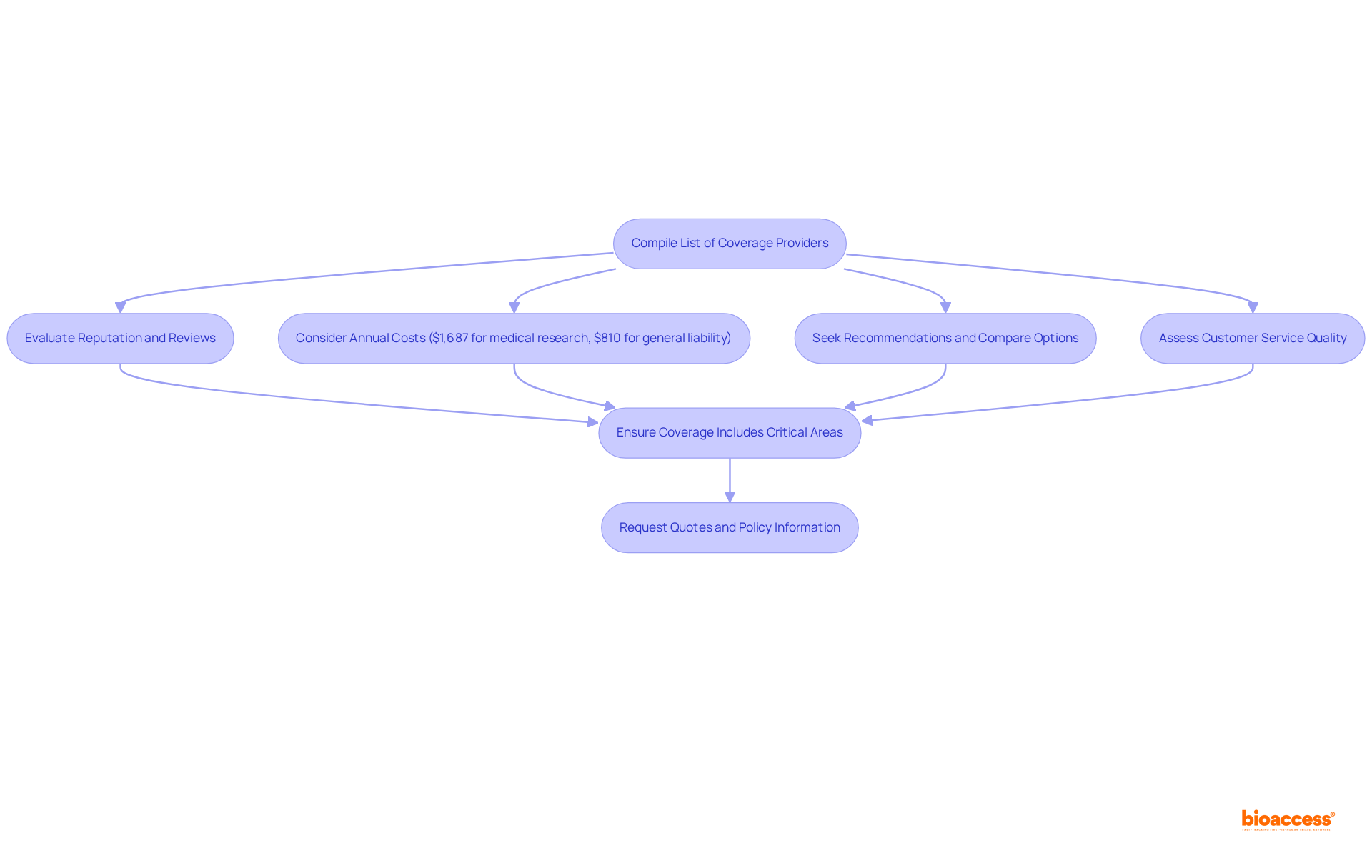
Begin your search by compiling a list of coverage providers that allow you to buy clinical trial insurance radiopharmaceuticals, particularly those specializing in research project protection. Seek out companies that boast a robust reputation within the industry, favorable client reviews, and a proven history of efficiently managing claims.
According to sector data, the average annual cost for medical research coverage is $1,687, while separate general liability protection averages $810 each year. These figures offer essential financial context for your decision-making process.
Engage with industry colleagues for recommendations and utilize online resources to compare insurance options and pricing. Furthermore, consider the provider's responsiveness and willingness to address your inquiries, as this can reflect their customer service quality.
It is imperative to ensure that when you buy clinical trial insurance radiopharmaceuticals, it encompasses critical areas such as clinical research liability and medical expenses for injured participants, given that adherence to regulatory requirements is vital for clinical studies.
Once you have narrowed down your options, request quotes and detailed policy information to support your decision-making process.
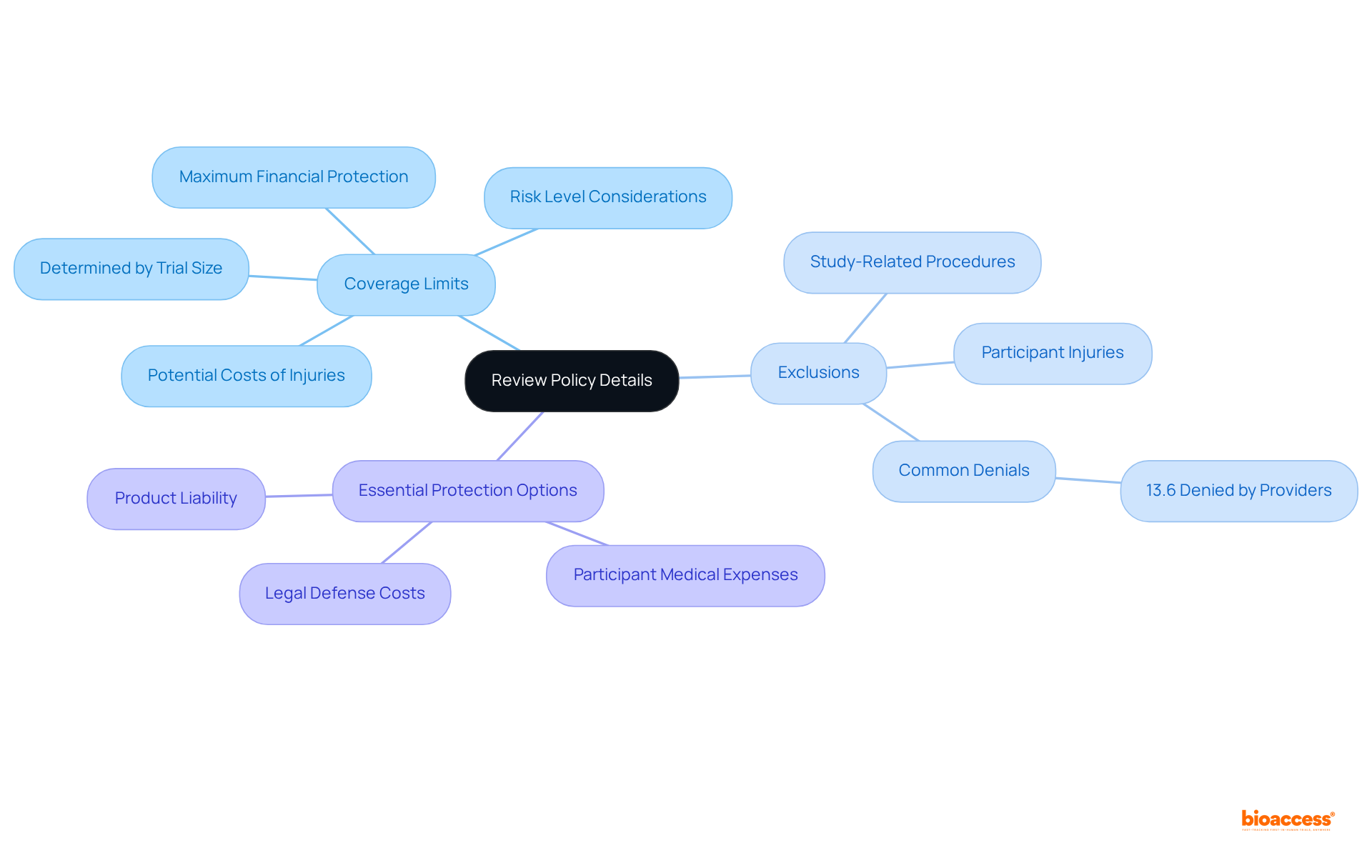
Upon receiving your policy documents, it is essential to conduct a meticulous review of each section, concentrating on coverage limits, exclusions, and any specific conditions that may apply. When you buy clinical trial insurance radiopharmaceuticals, pay particular attention to the definitions of covered events, ensuring they align with the unique risks related to your study.
As the National Institutes of Health emphasizes, 'buying clinical trial insurance radiopharmaceuticals is a crucial aspect of medical research, providing coverage for studies that offers financial security against unexpected occurrences and maintains the integrity of the study.'
Frequent exclusions in research-related coverage often involve particular study-related procedures and participant injuries that might not be included under standard policies. For instance, it has been reported that "13.6% of individuals were denied support for participation in clinical trials by their commercial providers," underscoring the significance of understanding your policy.
Look for essential protection options such as:
These can significantly enhance your safety. If any terms or conditions are unclear, do not hesitate to seek clarification from your coverage provider. This proactive strategy is vital for thoroughly comprehending your protection and making informed choices regarding your needs.
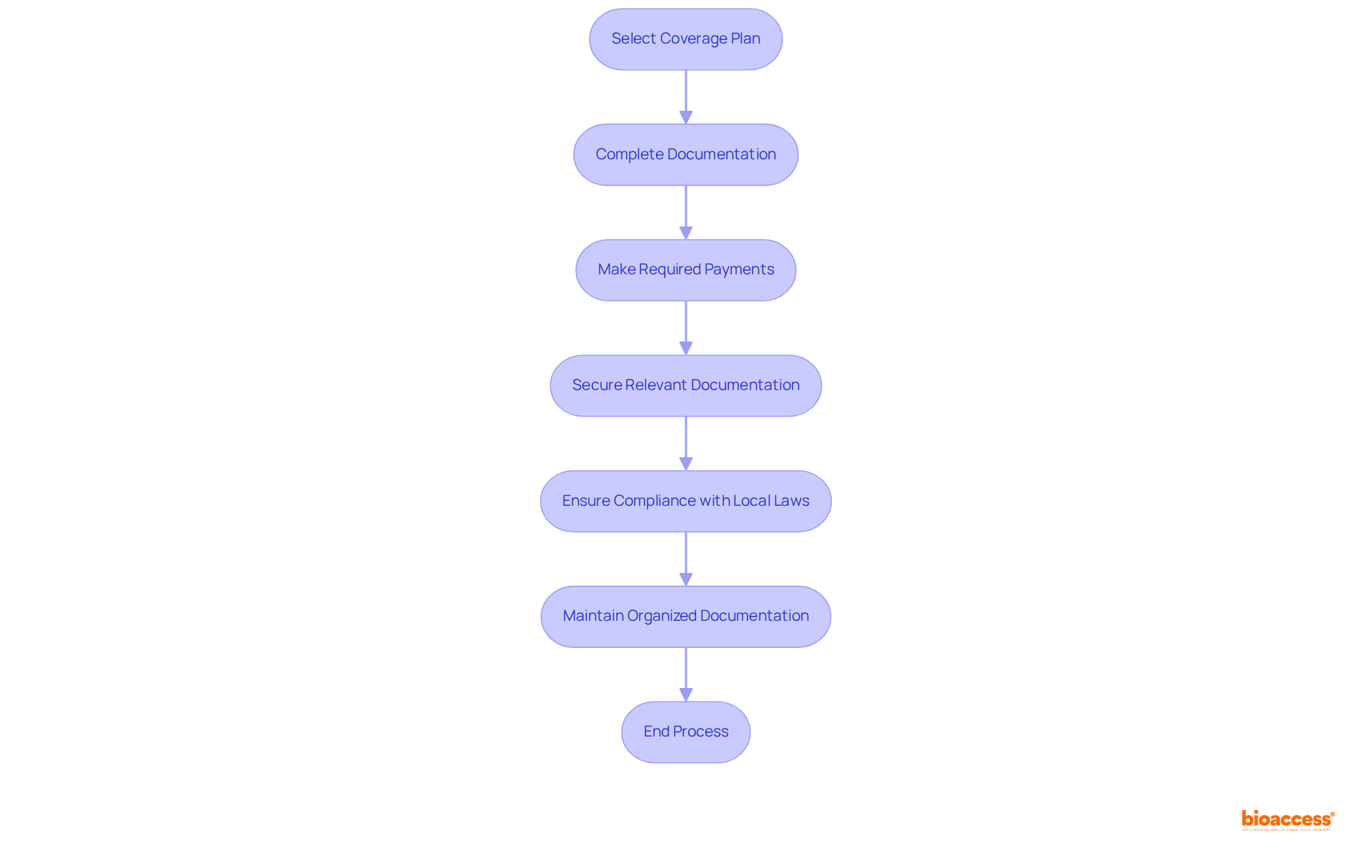
Upon selecting the appropriate coverage plan, finalize the acquisition by completing all necessary documentation and making the required payments. It is vital to secure all relevant documentation, including the policy certificate and any endorsements. Ensure your coverage adheres to local laws and meets the specific criteria established by the authorities overseeing your research study. Keeping organized and accessible documentation is essential, as it may be required during audits or inspections. By adhering to these regulations, you not only safeguard your experiment but also enhance the credibility of your research efforts. With your compliant insurance in place, you can confidently buy clinical trial insurance radiopharmaceuticals to advance your clinical trial initiatives.
Understanding the process of purchasing clinical trial insurance for radiopharmaceuticals is crucial for sponsors and researchers aiming to safeguard their studies against potential liabilities. This guide outlines the essential steps and considerations that empower individuals to navigate the complexities of insurance effectively, ensuring robust protection for their clinical trials.
Key insights include recognizing unique insurance needs specific to radiopharmaceuticals, such as:
Additionally, researching reputable insurance providers, meticulously reviewing policy details, and ensuring compliance with regulatory standards are vital components in securing the right coverage. By following these steps, stakeholders can mitigate risks and ensure their trials are financially secure.
Ultimately, investing in comprehensive clinical trial insurance is not merely a regulatory requirement; it is a strategic necessity that enhances the credibility and integrity of medical research. As the landscape of radiopharmaceutical studies continues to evolve, staying informed and proactive in securing appropriate insurance coverage will be pivotal in advancing clinical research initiatives.
What is clinical trial insurance and why is it important?
Clinical trial insurance is essential for protecting against potential liabilities associated with conducting clinical studies, including participant injuries, property damage, and legal costs from related claims.
What are the key types of coverage available in clinical trial insurance?
The key types of coverage include: - General Liability: Covers claims of bodily injury or property damage during the trial. - Professional Liability: Covers claims related to negligence or errors in the conduct of the trial. - Product Liability: Addresses risks associated with the use of investigational products, particularly relevant for radiopharmaceuticals.
What should I understand about terms like 'indemnity,' 'coverage limits,' and 'exclusions'?
Indemnity refers to the insurer's obligation to compensate for losses. Coverage limits define the maximum amount the insurer will pay for a claim, while exclusions detail specific circumstances or conditions not covered by the policy.
How do the average expenses for trial coverage vary?
The average expenses for trial coverage may vary significantly depending on the scope and nature of the study. Understanding these foundational elements is crucial for making informed decisions about purchasing clinical trial insurance.
What unique insurance needs should be considered for radiopharmaceuticals?
When assessing coverage needs for radiopharmaceuticals, consider: - The specific types of radiopharmaceuticals used - The likelihood of adverse reactions - The necessity to buy clinical trial insurance for radiopharmaceuticals - The regulatory obligations relevant to the trial's location
How does the scale of the trial affect insurance requirements?
Evaluating the trial's scale, including participant numbers and the complexity of procedures, helps clarify insurance requirements. For example, the oncology segment is particularly sensitive to risks due to the complexities of treatment protocols.
What services can help mitigate risks in clinical studies?
Engaging extensive clinical study management services can significantly diminish risks. These services include feasibility studies, compliance reviews, setup processes, import permits, project management, and reporting.
What is the anticipated growth of the global radiopharmaceuticals market?
The global radiopharmaceuticals market is anticipated to expand significantly, reaching USD 19.6 billion by 2034.