


The article delineates five essential steps for effectively purchasing technical file translation in Colombia. It underscores the critical importance of accuracy in translating healthcare documentation, which is vital for meeting regulatory requirements and ensuring effective communication with local authorities. This is supported by an emphasis on the necessity of certified translators, the thorough preparation of documents, and rigorous quality assurance processes. These measures are imperative to prevent compliance issues and to enhance the credibility of medical products within the Colombian market.
Navigating the complexities of the medical technology landscape in Colombia requires more than just innovative products; it demands precise communication through technical file translation. For Medtech companies, ensuring that all regulatory documents are accurately translated into Spanish is not merely a formality; it is a crucial step in fostering trust and compliance with local authorities like INVIMA. The stakes are high, as even minor translation errors can lead to significant delays and compliance issues.
How can companies effectively secure high-quality translations that meet stringent regulatory requirements while ensuring their products are positioned for success in a rapidly growing market?
For Medtech firms operating in Colombia, it is crucial to buy technical file translation Colombia to ensure that all paperwork related to healthcare instruments is accurately presented in Spanish. This process is not merely a regulatory obligation; it is vital for effective communication with local stakeholders, including INVIMA, the authority responsible for overseeing device approvals.
Accurate renditions significantly reduce the risk of misunderstandings that could result in compliance issues or delays in product approval. The case of Willie Ramirez, where the term 'intoxicado' was mistakenly interpreted as 'intoxicated,' underscores the severe consequences of interpretation errors in healthcare settings, highlighting the necessity for precision in healthcare communications.
Furthermore, meticulously executed adaptations bolster the credibility of both the product and the company, fostering trust among healthcare professionals and patients. As Colombia's healthcare product market continues to grow, with projections indicating a compound annual growth rate of 6.04% from 2025 to 2030, it is essential to buy technical file translation Colombia to ensure recognition of the significance of this conversion process for successful market entry and regulatory compliance.
In Colombia, to buy technical file translation Colombia, it is crucial to adhere to INVIMA's regulatory standards for the conversion of specialized documents to ensure successful medical equipment registration. All documents submitted must be in Spanish, and any foreign documents necessitate official rendering by a certified translator acknowledged by the Ministry of Foreign Affairs.
To ensure the technical file encompasses comprehensive details about the apparatus, including manufacturing processes and clinical data, it is essential to buy technical file translation Colombia to meet local regulations. Research indicates that approximately 30% of healthcare equipment registrations face delays due to conversion mistakes.
For instance, incorrect interpretations have resulted in compliance challenges that obstructed prompt approvals. Antonio Andrade, a pricing and market access strategy expert, emphasized that "grasping the subtleties of regulatory language is essential for navigating the complexities of registering healthcare products in Colombia."
Therefore, understanding and following these guidelines is paramount to streamline the approval process and avoid unnecessary setbacks.
Choosing a language service provider for your specialized document requires a thoughtful evaluation of several critical elements. Prioritize firms that specialize in healthcare equipment localization and possess a robust history in the sector. Ensure that the translators are certified and have substantial experience with documentation relevant to your product.
Additionally, it is crucial to select providers that implement rigorous quality assurance processes, including proofreading and editing by subject matter experts. According to industry standards, ISO 13485 certification is essential for quality management in healthcare product localization, ensuring compliance with regulatory requirements.
For assistance in locating certified translators in Colombia, resources such as Traductores.co can be invaluable when you want to buy technical file translation Colombia, ensuring your specialized file is handled by skilled professionals.
Industry leaders emphasize that the precise conversion of medical device documents is not only vital for regulatory compliance but also essential for safeguarding patient safety and ensuring effective use of medical devices by healthcare professionals.
To efficiently prepare your specialized file for conversion, begin by meticulously organizing all relevant documents, including product specifications, user manuals, and clinical trial data. It is imperative that these documents are comprehensive and devoid of errors prior to submission, as even minor inaccuracies can lead to significant misunderstandings during the conversion process. As emphasized, 'Translating specialized documents is a high-stakes undertaking. Getting it wrong can erode trust with global audiences.'
This diligent preparation not only streamlines the translation process but also guarantees that the final product complies with regulatory standards, ultimately enhancing the quality and reliability of your documentation.
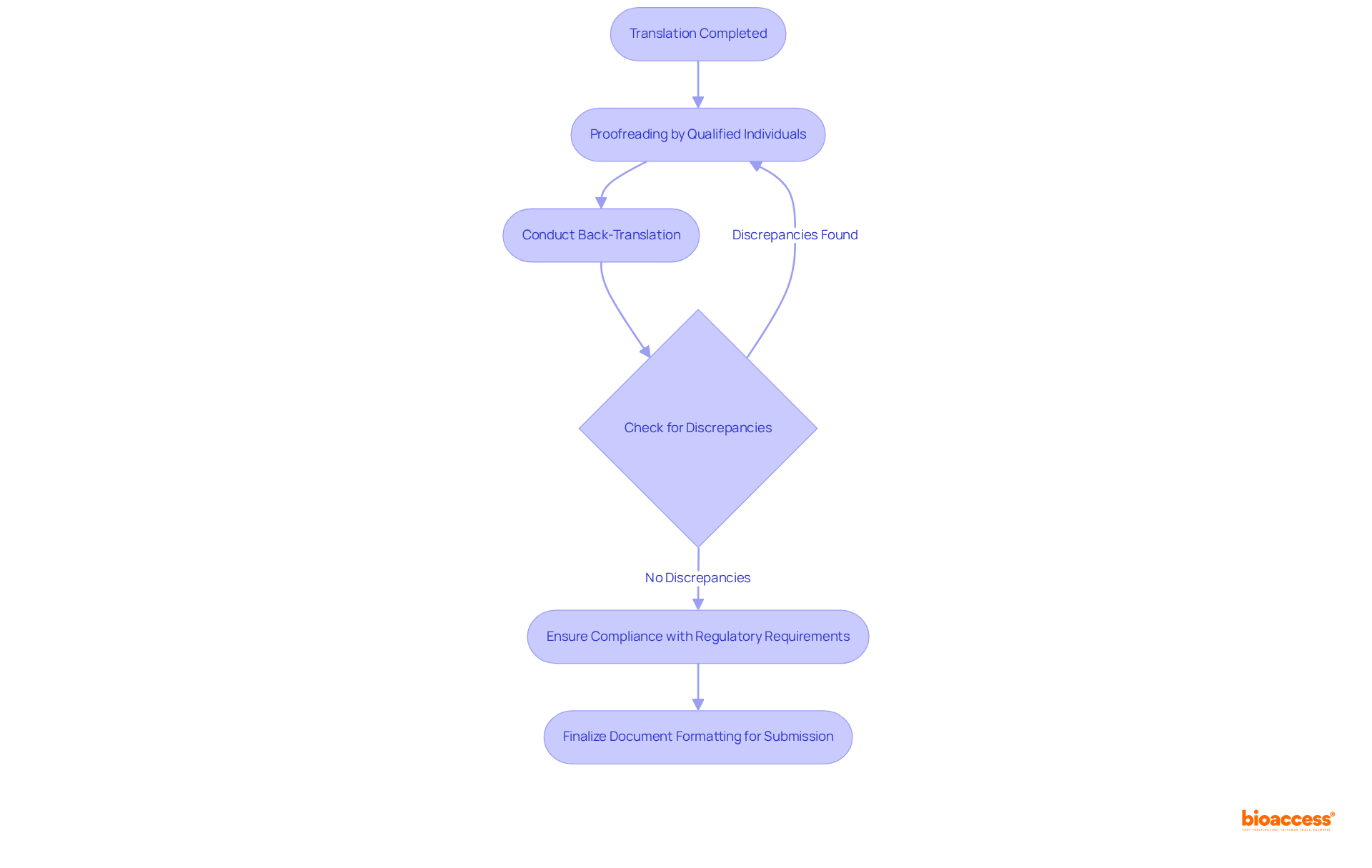
Once the specialized file has been translated, implementing a thorough review and quality assurance process is crucial. This process should encompass multiple rounds of proofreading by qualified individuals who are fluent in both languages and well-versed in the technical content.
Consider conducting a back-translation, where the translated document is rendered back into the original language by a different translator, to identify any discrepancies. Furthermore, ensure that the final document adheres to all regulatory requirements and is formatted correctly for submission to INVIMA.
This rigorous quality assurance process not only mitigates risks associated with translation errors but also ensures compliance with Colombian regulations when you buy technical file translation Colombia.
Understanding the nuances of technical file translation is essential for Medtech companies aiming to succeed in the Colombian market. Accurate translations are not merely a regulatory requirement; they are crucial for effective communication with local authorities and stakeholders. This process minimizes risks associated with misunderstandings, which can lead to compliance issues and delays in product approvals.
Key arguments underscore the importance of:
Each step, from grasping regulatory requirements to implementing thorough quality assurance processes, plays a pivotal role in ensuring that medical devices are accurately represented and compliant with local laws. By following these guidelines, companies can significantly enhance their chances of successful market entry and foster trust among healthcare professionals and patients.
In conclusion, the significance of technical file translation in Colombia is paramount. As the healthcare product market continues to expand, investing in high-quality translation services and adhering to best practices will facilitate regulatory compliance and ensure the safety and efficacy of medical devices. Companies are encouraged to prioritize this process to navigate the complexities of the Colombian market effectively, ultimately leading to better outcomes for both businesses and patients alike.
Why is technical file translation important for Medtech firms in Colombia?
Technical file translation is crucial for Medtech firms in Colombia to ensure that all healthcare instrument paperwork is accurately presented in Spanish, facilitating effective communication with local stakeholders like INVIMA and reducing the risk of misunderstandings that could lead to compliance issues or delays in product approval.
What are the consequences of inaccurate translations in healthcare?
Inaccurate translations can lead to severe consequences, such as compliance issues and delays in product approval. An example is the case of Willie Ramirez, where a misinterpretation of the term 'intoxicado' as 'intoxicated' highlighted the critical need for precision in healthcare communications.
How does technical file translation affect a company's credibility?
Meticulously executed translations enhance the credibility of both the product and the company, fostering trust among healthcare professionals and patients, which is essential for successful market entry.
What are the regulatory requirements for technical file translation in Colombia?
In Colombia, all documents submitted for medical equipment registration must be in Spanish. Foreign documents require official translation by a certified translator recognized by the Ministry of Foreign Affairs to comply with INVIMA's regulatory standards.
What details must be included in the technical file for medical equipment?
The technical file must encompass comprehensive details about the apparatus, including manufacturing processes and clinical data, to meet local regulations.
What percentage of healthcare equipment registrations face delays due to translation mistakes?
Research indicates that approximately 30% of healthcare equipment registrations experience delays due to conversion mistakes.
Why is understanding regulatory language important for registering healthcare products in Colombia?
Understanding the subtleties of regulatory language is essential for navigating the complexities of registering healthcare products in Colombia, as emphasized by pricing and market access strategy expert Antonio Andrade.