


The primary objective of this article is to deliver a comprehensive guide on the steps necessary to swiftly and efficiently obtain a trial import license in Mexico. It delineates the essential documentation, outlines the submission process, and addresses common challenges that applicants may face. Emphasizing that meticulous preparation and adherence to regulations are vital for a successful application, this guide serves as an indispensable resource for those navigating the complexities of the licensing process.
Navigating the complex landscape of clinical trials in Mexico demands a comprehensive understanding of regulatory requirements, particularly the critical necessity of obtaining a trial import license. This essential document not only guarantees compliance with stringent safety standards but also facilitates the ethical approval process, empowering researchers to import investigational products effectively.
However, the journey to secure this license is riddled with potential pitfalls and challenges. How can stakeholders streamline their application process and circumvent common mistakes that could impede their research timelines?
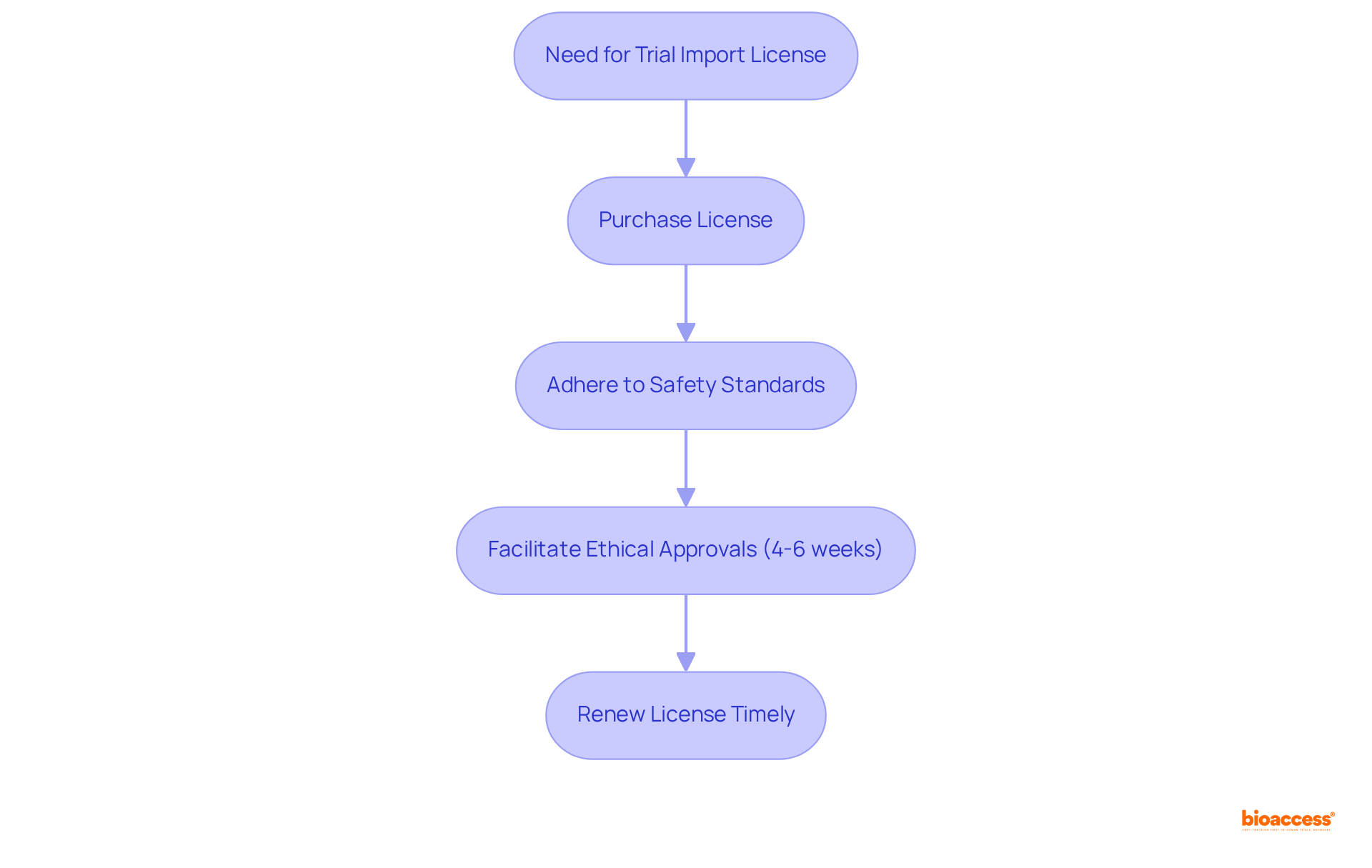
To conduct clinical trials in Mexico, you need to buy trial import license Mexico express for importing investigational products. Issued by the Federal Commission for the Protection against Sanitary Risks, this license guarantees that all imported products adhere to stringent safety and efficacy standards. Operating without this license can result in significant legal consequences, including potential fines and delays in clinical research.
Moreover, to buy trial import license Mexico express is instrumental in facilitating ethical approvals, typically granted within 4-6 weeks by bioaccess®, ensuring compliance with Mexican regulations that are crucial for the successful execution of trials. It is important to recognize that the license usually holds validity for a limited duration, making timely renewals critical to avoid disruptions in research activities.
As we look ahead to 2025, updates to regulations will continue to evolve, underscoring the necessity for stakeholders to remain informed about compliance requirements and their implications for trial import licenses. As Ana Criado, Director of Regulatory Affairs at Mahu Pharma, underscores, comprehending these guidelines is vital for achieving successful market entry in Latin America.
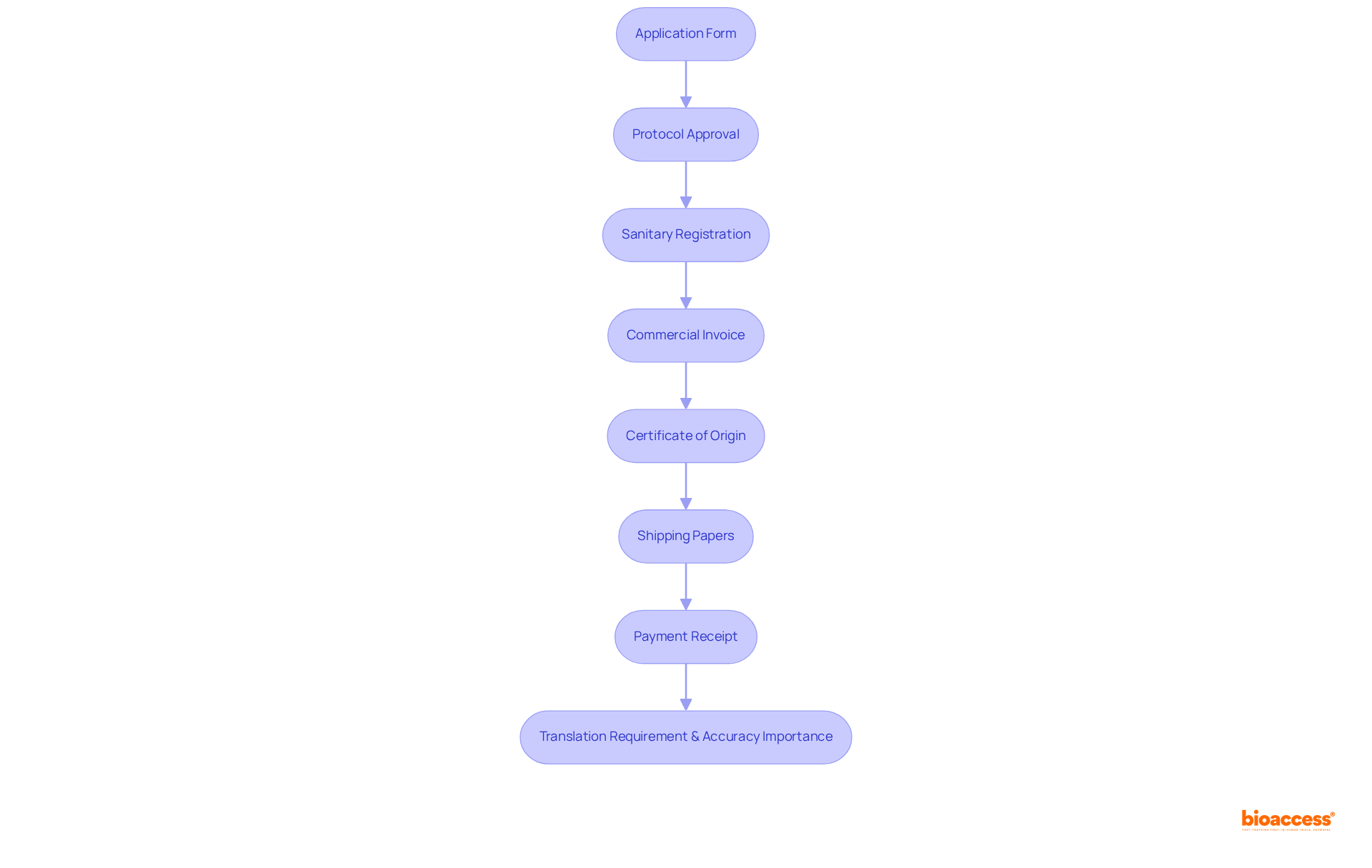
To successfully apply for a Trial Import License in Mexico, it is essential to compile several critical documents:
It is imperative that all documents are translated into Spanish, as COFEPRIS mandates submissions in the local language. Frequent documentation mistakes, like incomplete forms or absent approvals, can result in delays and complications in the application process. By ensuring comprehensive and precise documentation, applicants can simplify their submissions and improve the chances of a successful request when they buy trial import license mexico express. Furthermore, the regulatory authority has 10 working days to approve a REC registration request, highlighting the significance of prompt and thorough documentation in a competitive landscape where 3,282 clinical studies have been recorded in Mexico.
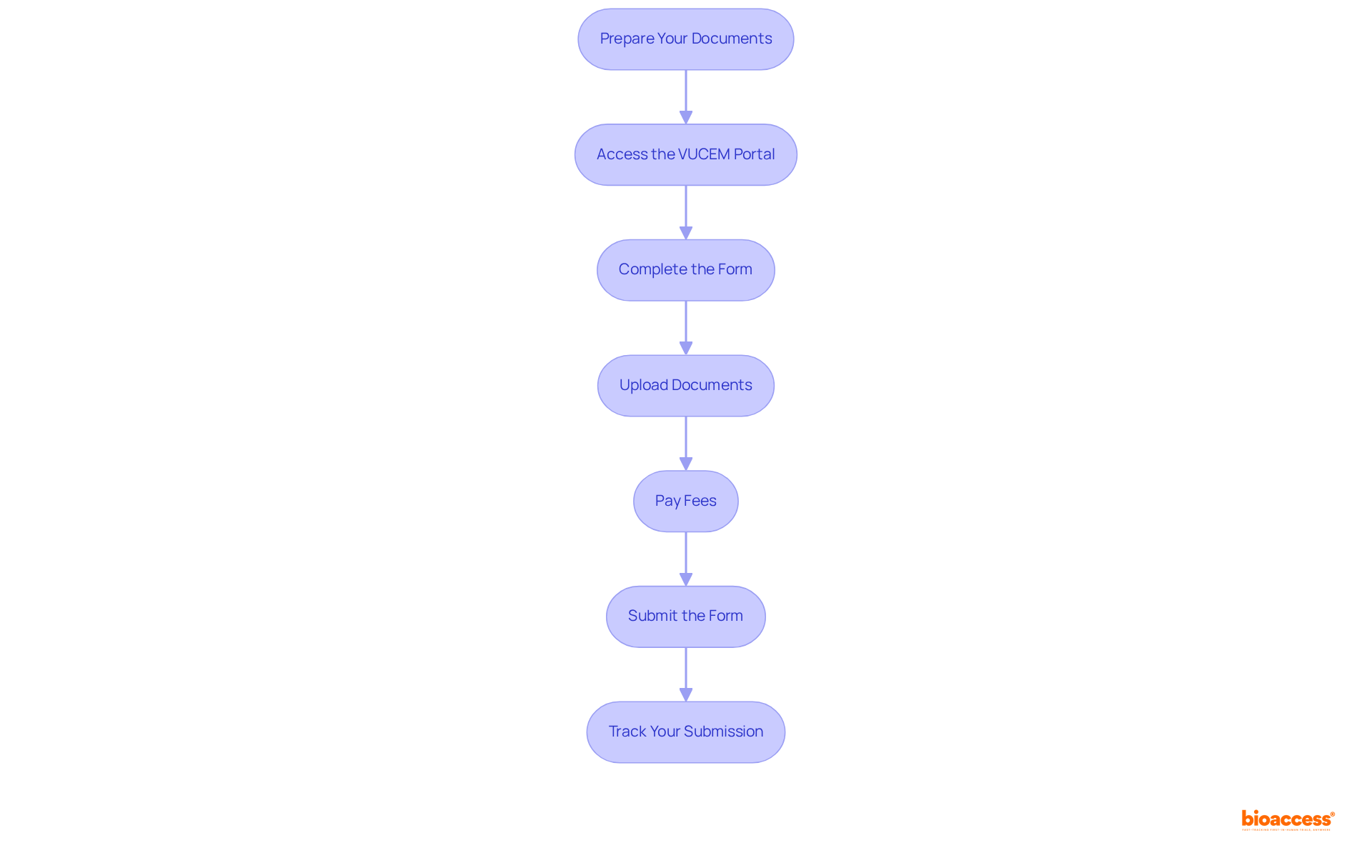
To successfully submit your Trial Import License application in Mexico, adhere to the following steps:
Success Rates and Best Practices: It is advisable to submit requests during the week prior to any scheduled maintenance on VUCEM to avoid delays. Historically, submissions accompanied by complete documentation and prompt replies to COFEPRIS inquiries have a higher success rate. By following these steps and best practices, you can effectively navigate the process to buy trial import license Mexico express, leveraging the VUCEM portal's capabilities to enhance your submission's success rate.
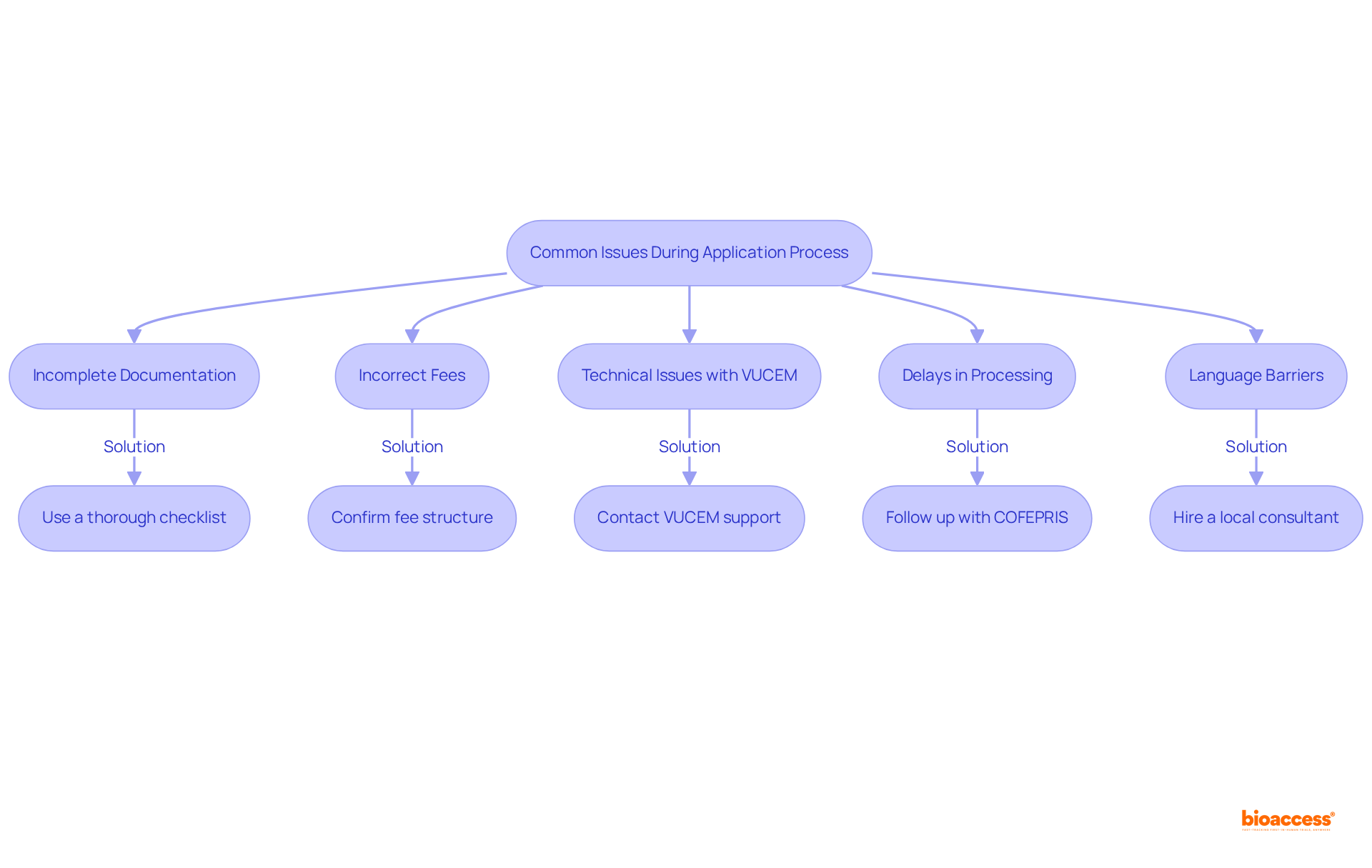
When applying for a Trial Import License, several common issues may arise that can hinder the process:
Incomplete Documentation: It is crucial to ensure that all required documents are submitted and formatted correctly. Absent signatures or translations can result in considerable hold-ups. A thorough checklist can help prevent these oversights.
Incorrect Fees: Familiarize yourself with the fee structure prior to payment. Insufficient payment or excessive payment can lead to processing holdups, so confirm the precise figures needed for your submission. Be aware that some customs brokers may include hidden costs such as extra documentation fees or storage fees, which can impact your overall budget.
Technical Issues with VUCEM: Should you encounter technical difficulties with the VUCEM system, promptly reach out to their support team for assistance. Keep a record of any error messages or issues for future reference.
Delays in Processing: If your request is taking longer than expected, it is advisable to follow up with COFEPRIS. Ask about the status of your request and resolve any pending issues that might be causing the delay. Understanding the average resolution time for common issues can help set realistic expectations.
Language Barriers: For those who are not fluent in Spanish, hiring a local consultant or translator can be beneficial. This support can facilitate document preparation and improve communication with regulatory authorities.
In light of the upcoming changes in the radiation protection act effective July 1, 2025, it is essential to stay informed about how these regulations may influence your submission process.
By proactively addressing these challenges and considering the additional factors mentioned, you can significantly improve your chances to buy a trial import license in Mexico express.
Acquiring a trial import license in Mexico represents a pivotal step for conducting clinical trials, ensuring adherence to the safety and efficacy standards established by the Federal Commission for the Protection against Sanitary Risks. This license not only facilitates the importation of investigational products but also serves as a cornerstone for maintaining the integrity and legality of clinical research operations. Grasping the complexities of obtaining this license is essential for stakeholders who aspire to adeptly navigate the regulatory landscape.
This article delineates a comprehensive strategy for securing a trial import license, highlighting the necessity of:
Key steps encompass:
By following these guidelines, applicants can significantly bolster their chances of success in obtaining the license.
Ultimately, the importance of a trial import license transcends mere compliance; it serves as a gateway to successful clinical trials and market entry in Mexico. Stakeholders are urged to stay informed about evolving regulations and to approach the licensing process with diligence and preparedness. By doing so, they can contribute to the advancement of healthcare research and innovation in the region, ensuring that investigational products meet the highest standards of safety and efficacy.
What is a trial import license in Mexico?
A trial import license in Mexico is a permit required for importing investigational products to conduct clinical trials. It is issued by the Federal Commission for the Protection against Sanitary Risks and ensures that all imported products meet safety and efficacy standards.
Why is a trial import license important for clinical trials in Mexico?
The trial import license is crucial because it guarantees compliance with safety and efficacy standards. Operating without this license can lead to legal consequences, including fines and delays in clinical research.
How long does it take to obtain ethical approvals after acquiring a trial import license?
Ethical approvals are typically granted within 4-6 weeks after obtaining the trial import license.
How long is a trial import license valid?
The trial import license usually holds validity for a limited duration, making timely renewals essential to avoid disruptions in research activities.
What should stakeholders be aware of regarding trial import licenses as we approach 2025?
Stakeholders should stay informed about evolving regulations and compliance requirements related to trial import licenses, as updates will continue to occur, impacting the execution of clinical trials in Mexico.
Who emphasizes the importance of understanding trial import license guidelines?
Ana Criado, Director of Regulatory Affairs at Mahu Pharma, emphasizes that comprehending these guidelines is vital for achieving successful market entry in Latin America.