


The article outlines five essential steps to successfully master first-in-human (FIH) clinical trials, emphasizing:
By underscoring the significance of strategic planning, adherence to ethical standards, and robust data management, it highlights how these components collectively enhance the likelihood of successful outcomes in clinical research. This comprehensive approach not only addresses the intricacies of FIH trials but also positions stakeholders to navigate the Medtech landscape effectively.
Navigating the intricate landscape of first-in-human (FIH) clinical trials presents a formidable challenge, yet it remains a crucial step in the journey of medical innovation. These trials act as a vital bridge between preclinical research and human testing, offering invaluable insights into the safety and efficacy of new treatments. However, the path to successful FIH trials is laden with regulatory hurdles, ethical considerations, and the urgent need for effective patient recruitment strategies.
How can researchers guarantee that their trials not only comply with regulatory standards but also engage participants effectively, paving the way for groundbreaking advancements in healthcare?
First-in human clinical trials are a pivotal phase in the development of new drugs, devices, or treatments, marking the crucial transition from preclinical research to human testing. Typically involving a small group of individuals, these studies are essential for assessing the safety, tolerability, and pharmacokinetics of investigational products. On average, FIH studies enroll between 20 to 80 participants, enabling researchers to gather essential preliminary data on human responses to the treatment.
The goals of FIH studies are multifaceted, concentrating on:
Successful FIH evaluations have led to substantial progress in therapies, with approximately 63%-70% of drugs succeeding in this initial phase. This success underscores the importance of robust preclinical evidence, which should be stronger for stable patients than for those with untreatable conditions.
Ethical considerations are vital in FIH studies, necessitating stringent informed consent procedures and emphasizing the safety of those involved. Experts in the field highlight that the responsibility of protecting subjects during this critical phase cannot be overstated. The ethical framework guiding these studies must ensure that participants are fully aware of the risks involved, fostering a transparent environment that respects their autonomy.
In summary, first-in human clinical trials are essential for advancing medical innovation, as they provide a foundation for subsequent phases of clinical research while balancing the need for scientific progress with ethical integrity.

Conducting first-in human clinical trials requires strict adherence to regulatory requirements, necessitating the submission of an Investigational New Drug (IND) application to the FDA or a Clinical Trial Application (CTA) to the EMA, depending on the jurisdiction. These submissions must include essential components such as comprehensive study protocols, informed consent forms, and safety data derived from preclinical studies. Furthermore, compliance with Good Clinical Practice (GCP) guidelines is crucial, as these standards ensure the ethical and scientific integrity of clinical studies. Engaging with regulatory bodies early in the planning phase can significantly streamline the approval process, enabling researchers to proactively identify and address potential challenges.
Colombia emerges as a compelling choice for FIH studies, offering cost reductions exceeding 30% compared to North America and Western Europe. The total review duration by IRB/EC and MoH (INVIMA) is notably efficient, taking only 90-120 days. The World Health Organization ranks Colombia's healthcare system at #22 globally, with its hospitals recognized as some of the finest in Latin America, assuring high-quality care during assessments. With a population exceeding 50 million and 95% covered by universal healthcare, patient recruitment in Colombia is robust. Moreover, the country provides significant R&D tax incentives, including a 100% tax deduction for investments in science and technology, a 25% tax discount, and a 50% future tax credit, making it an attractive destination for medical device startups.
The approval success rate for IND applications has experienced a marked increase, reflecting the FDA's commitment to facilitating innovative drug development. In 2023, the number of innovative drug IND applications accepted surged to 1,368, indicating a 30.78% year-on-year growth. This trend underscores the importance of comprehensive preparation and strategic planning in navigating the complexities of FIH evaluations. Bioaccess® can significantly aid in this process by providing expert services that connect startups with top-ranked clinical research sites, ensuring a smoother path to regulatory approval and successful study outcomes.

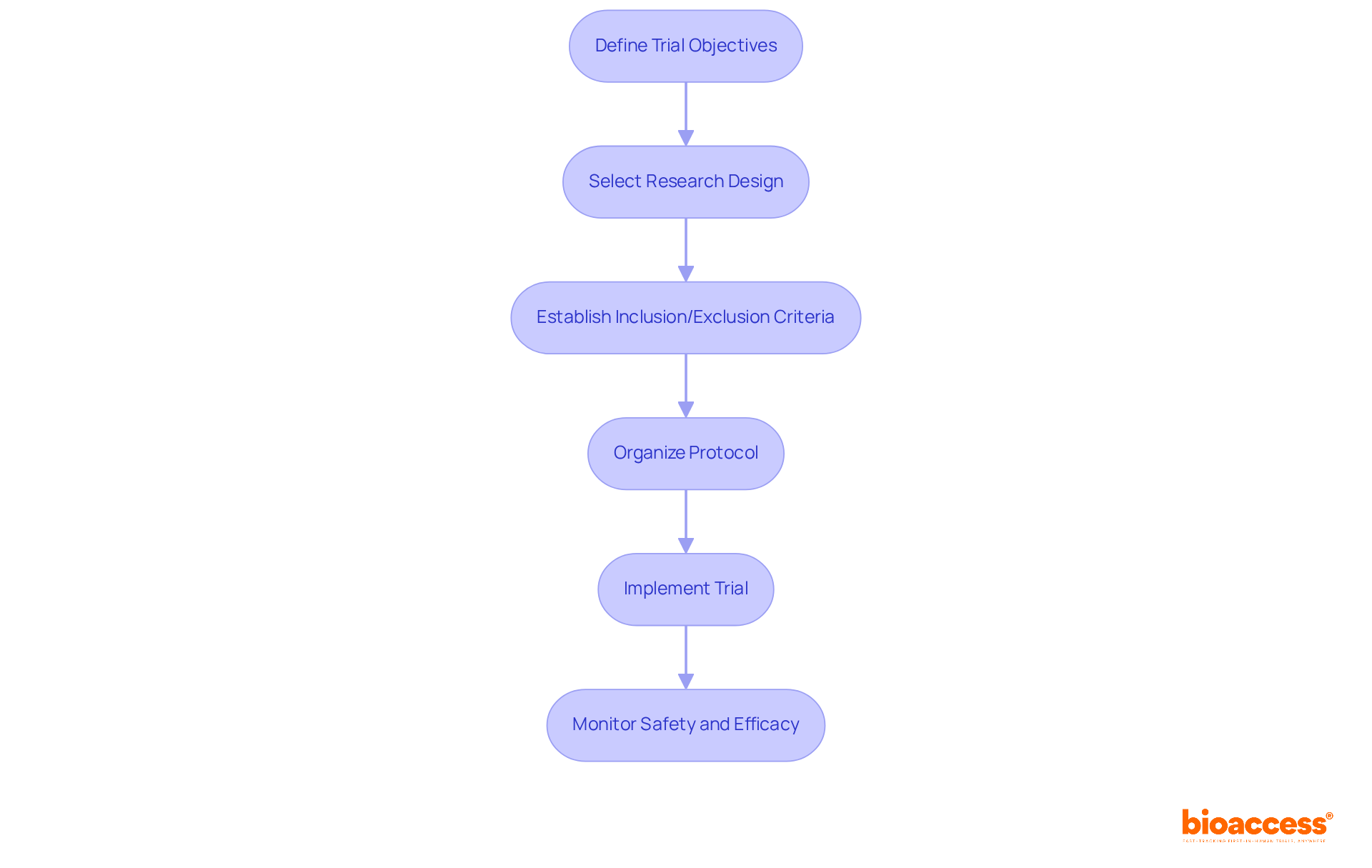
Designing first-in human clinical trials necessitates a strategic approach, beginning with a precise definition of trial objectives, which encompasses both primary and secondary endpoints. Selecting an appropriate research design is crucial; dose-escalation trials are often employed to effectively ascertain the maximum tolerated dose (MTD). Recent trends reveal that model-based and model-assisted methodologies are gaining prominence, providing improved accuracy in dose determination compared to traditional methods such as the 3 + 3 design, which frequently results in suboptimal dosing.
Establishing stringent inclusion and exclusion criteria is vital for participant selection, ensuring both safety and relevance to the research objectives. A meticulously organized protocol should delineate the methodology, data collection processes, and statistical analysis strategies, reflecting the latest advancements in first-in human clinical trials study design. For instance, the DEFINE study has significantly contributed to the establishment of guidelines that enhance transparency and reproducibility in first-in human clinical trials.
Once the design is finalized, the implementation phase involves recruiting participants and strictly adhering to the protocol. Ongoing oversight of safety and efficacy is imperative throughout the study period. As specialists emphasize, the choice of dose-escalation strategy can profoundly influence study results, making it essential to adopt approaches that align with the unique characteristics of the medication and the patient population. With bioaccess®, regulatory approval can be achieved in just 6-8 weeks, markedly faster than the typical 6-12 months in the US/EU. This expedited process facilitates the enrollment of treatment-naive cardiology or neurology cohorts 50% faster than Western sites, effectively addressing prevalent patient recruitment challenges. This comprehensive approach not only enables successful experimentation but also enhances the potential for significant clinical advancements.
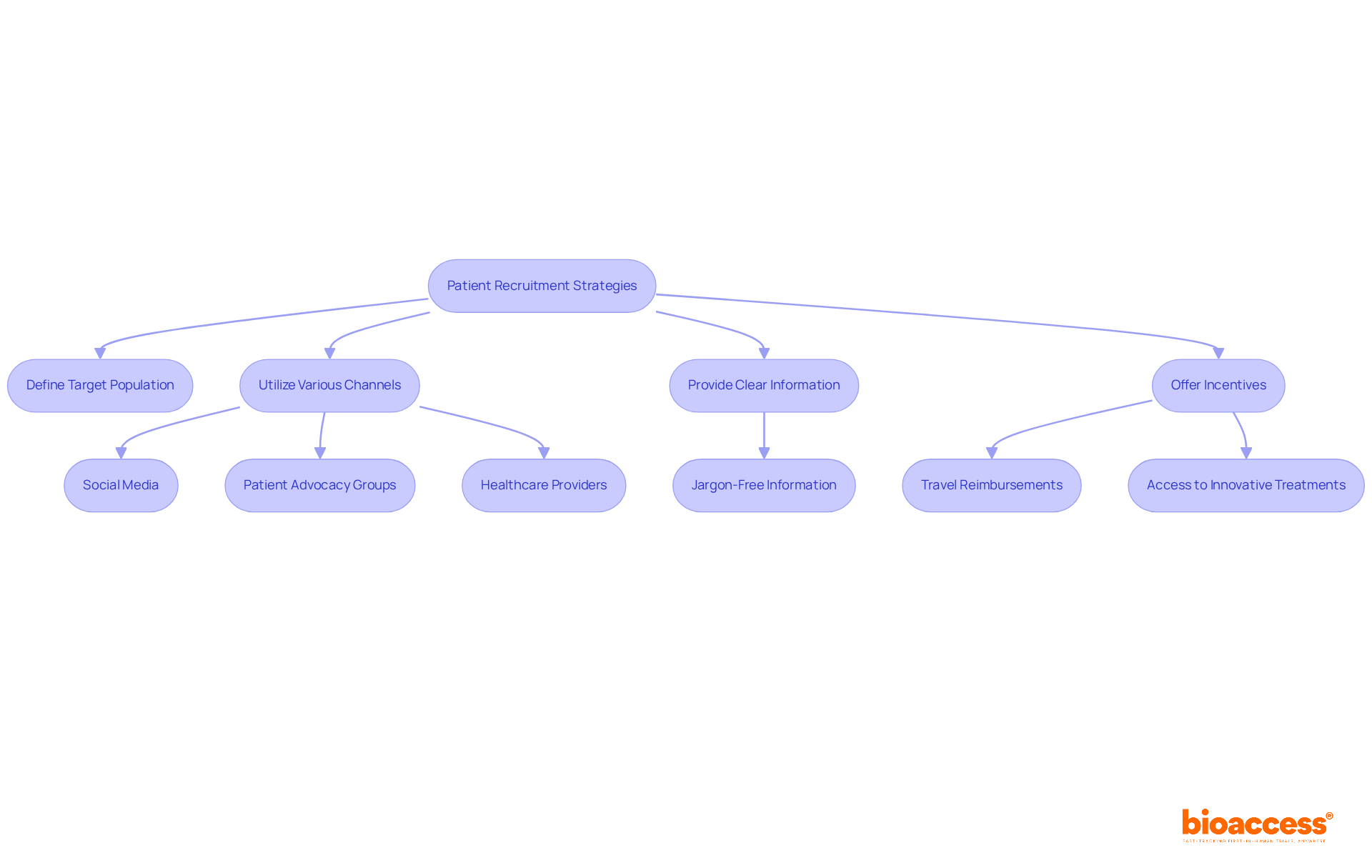
To successfully enlist individuals for first-in human clinical trials, a comprehensive recruitment approach is crucial. Clearly defining the target patient population based on the trial's inclusion and exclusion criteria is essential. A variety of channels should be utilized to connect with potential individuals, including:
With over 3.5 billion social media users globally, targeted digital campaigns can significantly enhance visibility and engagement with potential candidates.
Providing clear, jargon-free information about the trial—covering its purpose, procedures, and potential benefits—can alleviate concerns and foster interest. Research indicates that patient motivation often stems from dissatisfaction with current treatment options, making it crucial to communicate how participation may offer new solutions.
Incentives such as travel reimbursements or access to innovative treatments can further encourage participation, addressing the travel burdens that disproportionately affect patients from lower-income areas. Keeping open channels of communication during the recruitment process not only fosters trust but also improves retention, as regular updates and check-ins can keep candidates engaged and informed.
Notably, partnerships like that of GlobalCare Clinical Trials and bioaccess™ have demonstrated the effectiveness of leveraging local expertise to enhance recruitment efforts. Their partnership in Colombia has led to over a 50% decrease in clinical study subject recruitment time and a retention rate surpassing 95%. By applying these strategies, clinical study managers can enhance recruitment rates, which are essential for the success of first-in human clinical trials, as average participant recruitment rates can be difficult to attain without a careful approach. Additionally, bioaccess™ brings extensive expertise in managing various types of clinical studies, further enhancing the effectiveness of these recruitment strategies.
Effective monitoring and management of first-in human clinical trials hinge on several essential practices. A robust data management system is vital for effectively tracking individual progress, adverse events, and data collection. Recent evaluations reveal that while mild adverse events are frequent, serious negative occurrences are rare, with only 0.31% of individuals experiencing severe incidents.
Regular reviews of data for accuracy and completeness, alongside interim analyses, are crucial for assessing safety and efficacy. Implementing a risk-based monitoring approach enables focused resource allocation on high-risk areas, ensuring compliance with regulatory requirements. Furthermore, maintaining open lines of communication with the research team, regulatory bodies, and ethics committees is essential for promptly addressing any concerns.
By fostering a culture of transparency and accountability, the overall success of the experiment can be significantly enhanced. Successful case studies illustrate that organizations employing advanced data management systems not only streamline processes but also enhance participant safety and data integrity, ultimately leading to more reliable trial outcomes.

Mastering first-in-human clinical trials is crucial for advancing medical research and innovation. These trials serve as the critical bridge between preclinical studies and human testing, providing invaluable insights into the safety and efficacy of new treatments. By understanding the complexities involved, including regulatory requirements, strategic design, effective patient recruitment, and diligent monitoring, researchers can significantly enhance their chances of success.
Key arguments presented highlight the importance of a well-structured approach to conducting these trials. Adhering to stringent regulations and ethical considerations, implementing robust patient recruitment strategies, and employing effective data management systems are vital steps in ensuring that trials are conducted efficiently and ethically. The potential for breakthroughs in medical science hinges on the ability to execute these trials successfully, making mastery of these steps not just beneficial but essential.
In conclusion, the significance of first-in-human clinical trials cannot be overstated. They represent a pivotal moment in drug development that balances the pursuit of innovation with the utmost care for participant safety. By embracing best practices and leveraging expert support, stakeholders can navigate the complexities of these trials, ultimately leading to advancements that may transform patient care and treatment options. Engaging in this rigorous process is not merely a procedural necessity; it is a commitment to ethical medical progress that can change lives.
What are first-in-human (FIH) clinical trials?
First-in-human clinical trials are a critical phase in drug, device, or treatment development, marking the transition from preclinical research to human testing. They typically involve a small group of 20 to 80 participants to assess safety, tolerability, and pharmacokinetics of investigational products.
What are the main goals of FIH studies?
The main goals of FIH studies include determining the maximum tolerated dose, identifying potential side effects, and establishing a safety profile for the investigational product.
What is the success rate of FIH evaluations?
Approximately 63% to 70% of drugs succeed in the first-in-human phase, highlighting the importance of robust preclinical evidence.
What ethical considerations are involved in FIH studies?
Ethical considerations in FIH studies include stringent informed consent procedures and ensuring the safety of participants. It is essential that participants are fully aware of the risks involved to foster transparency and respect their autonomy.
What regulatory requirements must be met for conducting FIH trials?
FIH trials require the submission of an Investigational New Drug (IND) application to the FDA or a Clinical Trial Application (CTA) to the EMA, including study protocols, informed consent forms, and safety data from preclinical studies. Compliance with Good Clinical Practice (GCP) guidelines is also crucial.
Why is Colombia considered an attractive location for FIH studies?
Colombia is appealing for FIH studies due to cost reductions exceeding 30% compared to North America and Western Europe, efficient review durations of 90-120 days, a strong healthcare system, robust patient recruitment, and significant R&D tax incentives.
What is the current trend in IND application approvals?
The approval success rate for IND applications has increased, with 1,368 innovative drug IND applications accepted in 2023, reflecting a 30.78% year-on-year growth, indicating the FDA's commitment to facilitating drug development.
How can Bioaccess® assist in the FIH trial process?
Bioaccess® can provide expert services that connect startups with top-ranked clinical research sites, aiding in the regulatory approval process and ensuring successful study outcomes.