


The article delineates five essential steps for navigating medical device regulation in Health Canada. These steps encompass:
Each step underscores the critical necessity of adhering to safety, effectiveness, and quality standards, all supported by comprehensive guidelines and the pivotal role of regulatory bodies. This structured approach not only facilitates successful market entry but also ensures ongoing compliance for medical device manufacturers.
Navigating the complex landscape of medical device regulation in Canada is crucial for ensuring the safety and efficacy of healthcare products. With a market poised for significant growth, understanding the intricacies of Health Canada’s regulatory framework empowers manufacturers and innovators alike.
However, the challenge lies in deciphering the multifaceted classification systems and compliance requirements that govern medical devices.
How can stakeholders effectively maneuver through these regulatory hurdles to bring their innovations to market while safeguarding patient outcomes?
Healthcare instruments encompass a wide array of items employed for health-related purposes, including diagnostic tools, therapeutic apparatus, and surgical instruments. Their significance is underscored by their capacity to enhance patient outcomes, elevate the quality of care, and enable innovative treatments. Understanding the categorization of healthcare instruments, their intended applications, and the potential hazards associated with them is essential for navigating the medical device regulation Health Canada landscape. This comprehension is vital for recognizing the importance of adhering to medical device regulation Health Canada, which ensures that products are safe and effective for public use.
Moreover, utilizing comprehensive clinical trial management services, such as those provided by Bioaccess, can greatly simplify this process. Bioaccess delivers:
These services are crucial for Medtech, Biopharma, and Radiopharma startups aiming to effectively navigate the complexities of health product regulations. Their expertise not only streamlines regulatory compliance but also fosters innovation and growth in the sector.

Health Canada plays a crucial role in overseeing healthcare instruments, ensuring their safety, effectiveness, and quality through the Medical Devices Regulations (MDR). This framework delineates the requirements for classification, licensing, and post-market surveillance of medical devices under the medical device regulation health canada. Key regulatory bodies, such as the Therapeutic Products Directorate (TPD) and the Medical Devices Bureau (MDB), are integral to this oversight process. Understanding their functions is essential for compliance.
The Risk-Based Classification System is a cornerstone of the medical device regulation Health Canada guidelines, categorizing products according to their potential risks to patients. For instance, Class I items, considered low-risk, require a Medical Device Establishment License, whereas higher-risk Class II, III, and IV items necessitate more stringent licensing and post-market obligations. Recent improvements to the oversight structure, including compulsory reporting for healthcare equipment incidents under Vanessa's Law, demonstrate Health Canada's dedication to enhancing safety and transparency in the context of medical device regulation health canada.
Statistics indicate that in 2022, the Canadian medical device market was valued at approximately US$6.8 billion, with an expected annual growth rate of 5.4% until 2028. This growth underscores the significance of effectively maneuvering through the compliance environment. Insights from regulatory experts emphasize the importance of understanding these evolving regulations to ensure compliance and successful market entry. By familiarizing yourself with these guidelines, you can better navigate the intricacies of health product regulation in Canada.
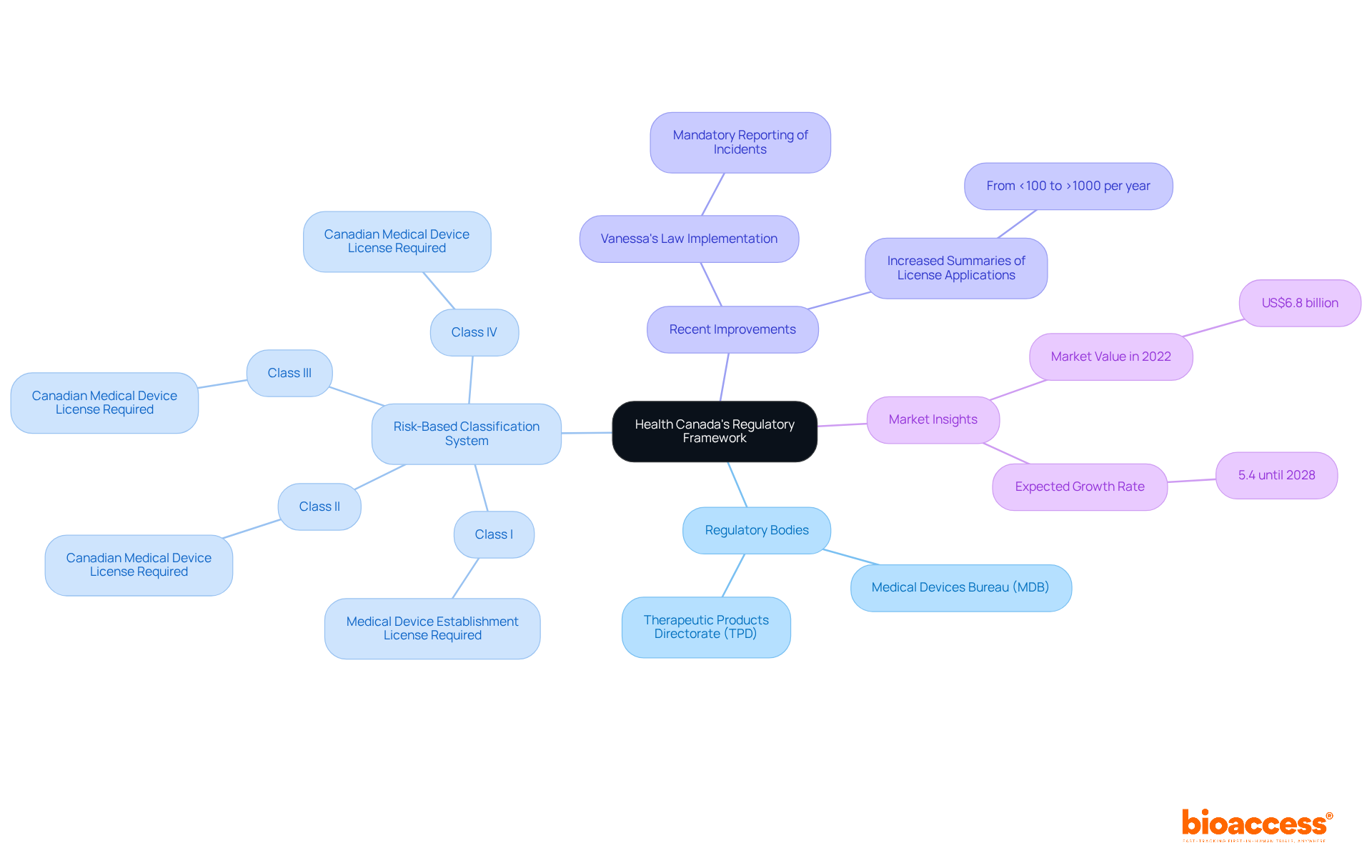
In Canada, medical device regulation Health Canada categorizes medical instruments into four classes (I, II, III, and IV) according to their associated risk levels. Class I items, which pose the lowest risk, require minimal oversight, while Class IV items, representing the highest risk, are subject to rigorous scrutiny. This classification process entails assessing the intended use of the equipment, the technology it utilizes, and the possible risks it may present to users. Health Canada offers detailed guidance documents on medical device regulation health canada that specify the criteria for classification, essential for identifying the suitable pathway for approval.
Precise categorization is crucial, as it directly affects the required regulatory actions and schedules for launching your product. For instance, Class II products may require a Medical Product License (MDL) and proof of safety and effectiveness, while Class III and IV items necessitate more extensive clinical data and regulatory review. Grasping these classifications not only guarantees adherence to medical device regulation health canada but also enhances the safety and efficacy of healthcare products available to Canadians. As you navigate these classifications, consider how they impact your clinical research objectives and the overall landscape of Medtech.

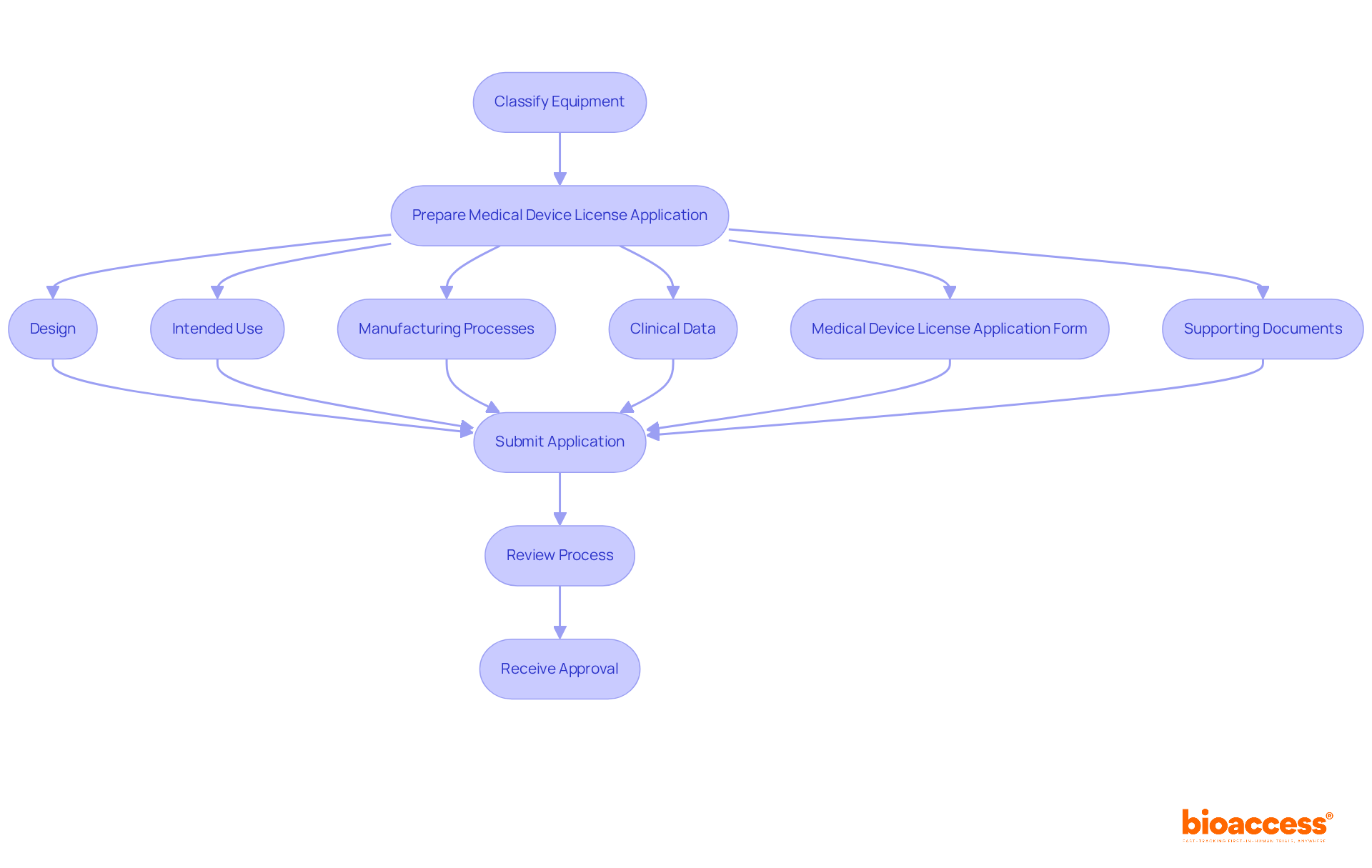
Once your equipment is classified, the next step is to prepare and submit a medical equipment license application. This application must include detailed information about the apparatus, encompassing its design, intended use, manufacturing processes, and clinical data demonstrating its safety and effectiveness. Ensure that all documentation is complete and adheres to the medical device regulation Health Canada submission guidelines. Utilize the Medical Device License Application Form and include any necessary supporting documents, such as risk assessments and clinical trial results.
Notably, a well-prepared application can significantly expedite the review process, which currently averages approximately three weeks for Class II devices, exceeding the Bureau's 15-day policy target. Processing times for Class III and IV applications are now approximately 60 days longer than the Bureau's established review targets due to increased complexity and volume. According to compliance experts, the significance of precise and thorough information in applications cannot be emphasized enough, as it directly impacts the chances of obtaining a Substantially Equivalent decision from the FDA, with 15 percent of 510(k) applications not securing this approval in the past year. As a prominent regulatory specialist noted, 'Regulatory adherence is not merely an obstacle to be overcome; it’s a continuous dedication to patient safety and product quality.' By adhering to these guidelines and leveraging insights from successful submissions in the context of medical device regulation Health Canada, you can enhance your chances of a smooth approval process.
Additionally, remember that a separate Medical Device Licence Fee Form is required for each application, and the application must include a copy of the device label along with all related labelling and instructions. It is also crucial to have the application signed by an authorized senior official to ensure adherence. Implementing a robust document and quality management system (QMS) can help organize and store documents efficiently, further supporting your application process. Moreover, utilizing extensive clinical trial management services, like those provided by bioaccess, can assist with feasibility studies, site selection, regulatory reviews, and project management, guaranteeing that your application is well-supported and follows all required regulations.
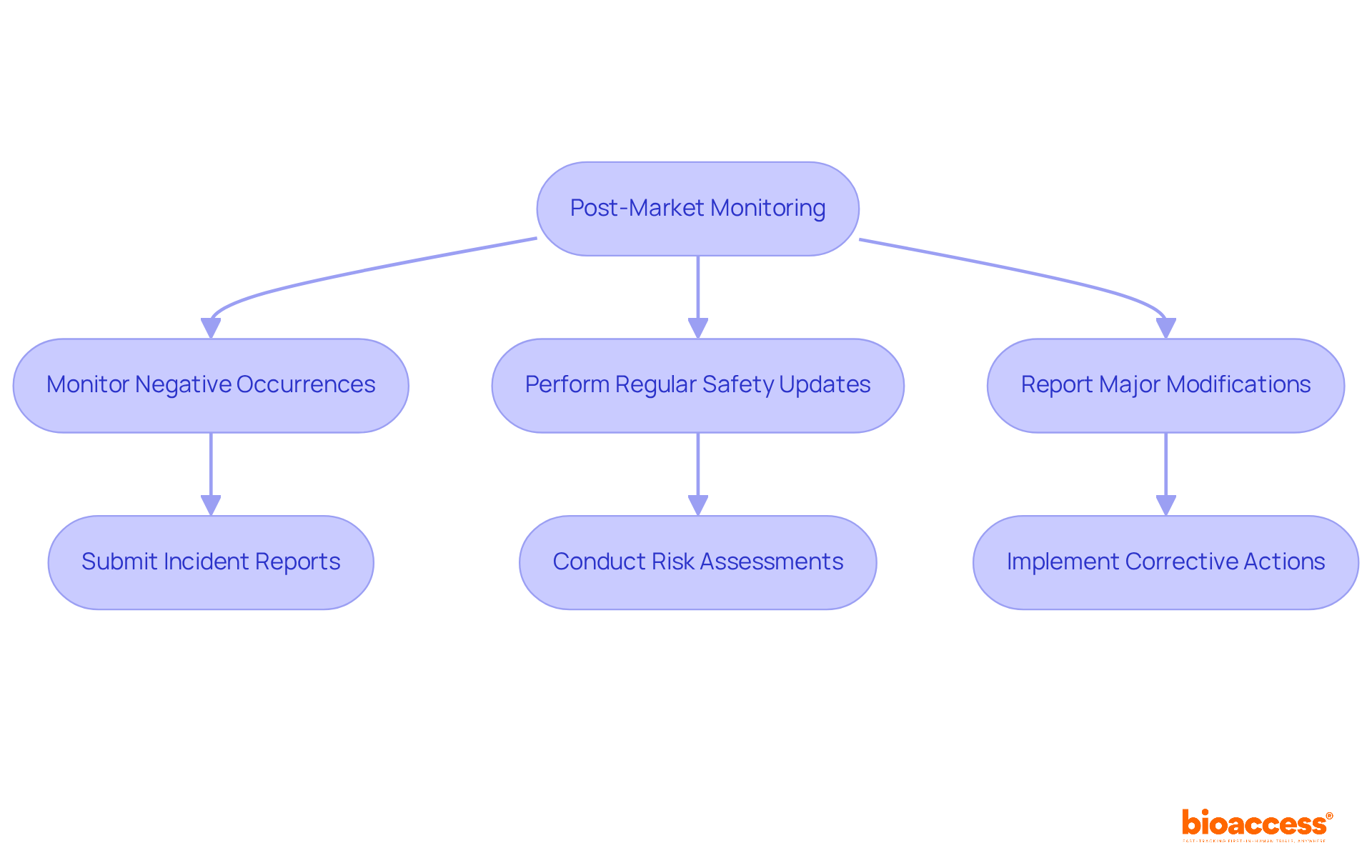
Following approval, it is essential for manufacturers to uphold compliance with the medical device regulation Health Canada through diligent monitoring and reporting. Post-market monitoring is a required procedure that allows for the detection of possible problems with medical equipment. This entails:
For instance, in 2017, Health Canada received approximately 22,000 Medical Devices Incident Reports, underscoring the importance of effective monitoring systems.
Establishing a robust quality management system is vital for ensuring compliance and facilitating timely reporting. Corrective and preventive actions (CAPA) are indispensable for addressing identified issues promptly. By actively evaluating your equipment's performance in the market, you can tackle issues swiftly, thus preserving the confidence of oversight authorities and patients alike. Experts emphasize that comprehensive post-market surveillance is crucial for patient well-being and reinforces market authorization, making it a cornerstone of responsible medical device regulation Health Canada.
Additionally, it is crucial for manufacturers to adapt these practices to meet local regulatory requirements, ensuring compliance across different jurisdictions.
Navigating the landscape of medical device regulation in Canada is an essential endeavor for ensuring the safety and effectiveness of healthcare instruments. By comprehensively understanding the regulatory framework established by Health Canada—encompassing the classification of devices and the necessary compliance measures—stakeholders can successfully introduce innovative solutions to the market while prioritizing patient safety.
This article delineates five crucial steps:
Each step underscores the necessity of adhering to established guidelines and the advantages of utilizing specialized services to streamline the process. Notably, the emphasis on thorough documentation and post-market monitoring highlights the ongoing commitment to quality and safety within the medical device industry.
Ultimately, the significance of medical device regulation cannot be overstated. It not only safeguards patients but also cultivates innovation within the healthcare sector. By remaining informed and proactive in compliance efforts, manufacturers can contribute to improved health outcomes and elevate the overall quality of care in Canada. Embracing these regulatory practices is imperative for any organization aiming to thrive in the evolving landscape of medical technology.
What are healthcare instruments, and why are they important?
Healthcare instruments include diagnostic tools, therapeutic apparatus, and surgical instruments used for health-related purposes. They are important because they enhance patient outcomes, improve the quality of care, and enable innovative treatments.
What is the role of Health Canada in regulating medical devices?
Health Canada oversees healthcare instruments to ensure their safety, effectiveness, and quality through the Medical Devices Regulations (MDR). This includes requirements for classification, licensing, and post-market surveillance of medical devices.
What are the key regulatory bodies involved in medical device oversight in Canada?
The key regulatory bodies include the Therapeutic Products Directorate (TPD) and the Medical Devices Bureau (MDB), which are essential for ensuring compliance with medical device regulations.
How does the Risk-Based Classification System work in Canada?
The Risk-Based Classification System categorizes medical devices based on their potential risks to patients. Class I devices are low-risk and require a Medical Device Establishment License, while higher-risk Class II, III, and IV devices require more stringent licensing and post-market obligations.
What is Vanessa's Law, and how does it affect medical device regulation?
Vanessa's Law mandates compulsory reporting for healthcare equipment incidents, reflecting Health Canada's commitment to enhancing safety and transparency in medical device regulation.
What are the statistics regarding the Canadian medical device market?
In 2022, the Canadian medical device market was valued at approximately US$6.8 billion, with an expected annual growth rate of 5.4% until 2028.
How can companies navigate the complexities of health product regulations in Canada?
Companies can utilize comprehensive clinical trial management services, such as those provided by Bioaccess, which offer feasibility analyses, site selections, compliance assessments, trial arrangements, import licenses, project oversight, and reporting to streamline regulatory compliance and foster innovation.