


The primary components of a master device record, essential for success in the Medtech industry, encompass:
Integrating these components is critical, as it not only ensures regulatory compliance but also significantly enhances product quality and safety. This integration ultimately contributes to the efficiency and success of clinical research and medical device development. By recognizing the importance of these elements, stakeholders can navigate the complexities of the Medtech landscape more effectively.
The landscape of clinical research within the Medtech industry is undergoing rapid transformation, with the master device record (MDR) emerging as a pivotal element in ensuring compliance and efficiency. A well-structured MDR not only streamlines documentation but also accelerates the approval process for innovative medical products, ultimately enhancing patient outcomes.
However, the complexities of regulatory requirements, coupled with the challenges posed by manual management, raise a critical question: how can organizations effectively navigate the intricacies of maintaining an effective device master record?
This article delves into the essential components of a master device record, offering insights into best practices that can transform compliance challenges into opportunities for success.
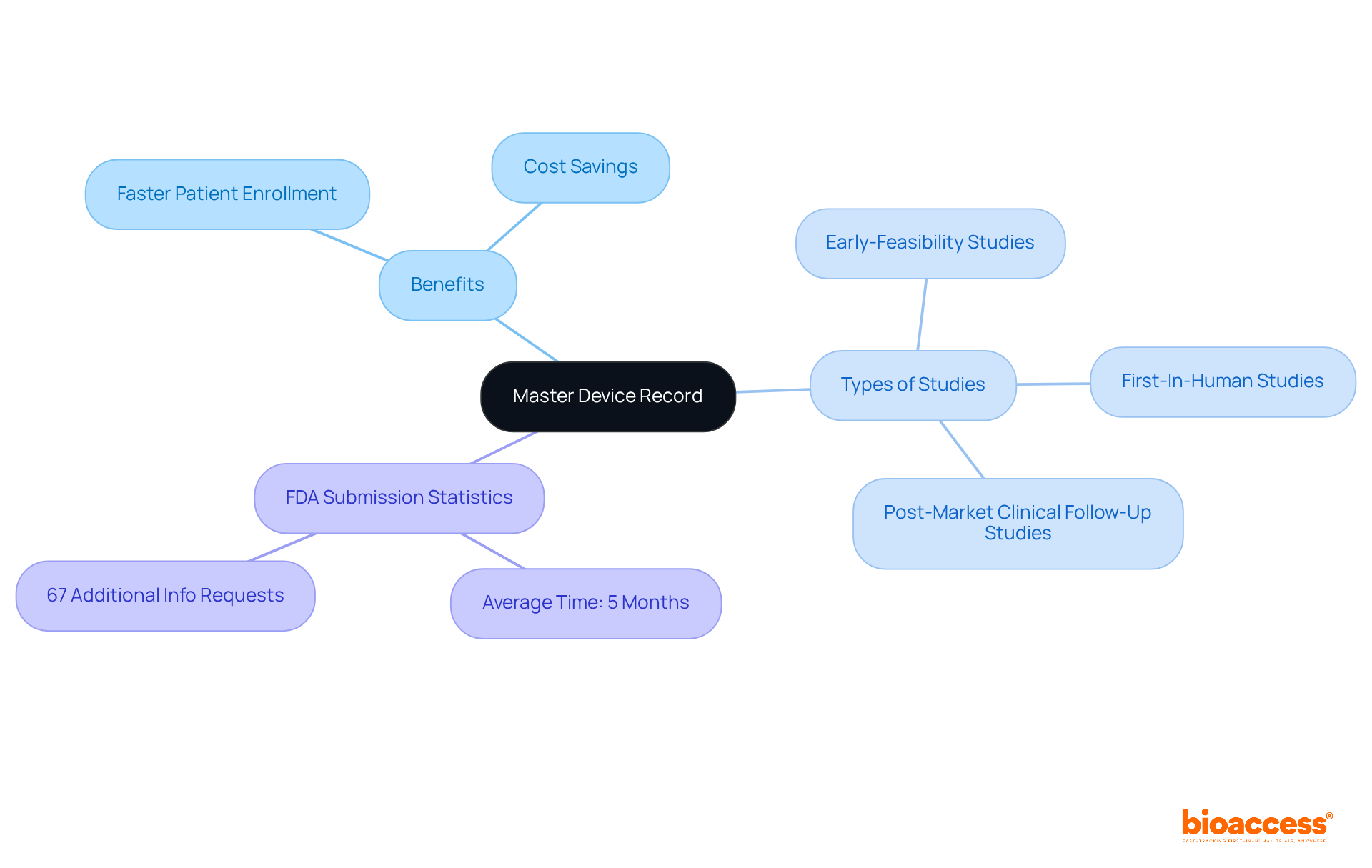
The successful implementation of the master device record is essential for Medtech companies aiming to accelerate clinical research in Latin America. By centralizing and organizing crucial documentation, bioaccess® enhances approval processes, significantly reducing time to market for innovative medical products.
With a proven track record spanning over 20 years in Medtech, bioaccess® specializes in managing clinical trials, including:
Statistics indicate that the average time for FDA 510(k) submissions is approximately five months, with 67 percent resulting in requests for additional information, which can further delay approvals. In contrast, a well-maintained master device record streamlines this process, ensuring that strict compliance standards are met. This not only expedites clinical trials but also enhances patient outcomes and fosters advancements in medical knowledge.
Industry leaders underscore that the master device record is crucial for maintaining efficiency in clinical trials, reinforcing the necessity of centralized documentation within the Medtech landscape. bioaccess® offers a tailored approach that achieves patient enrollment 50% faster and saves $25K per patient with FDA-ready data.
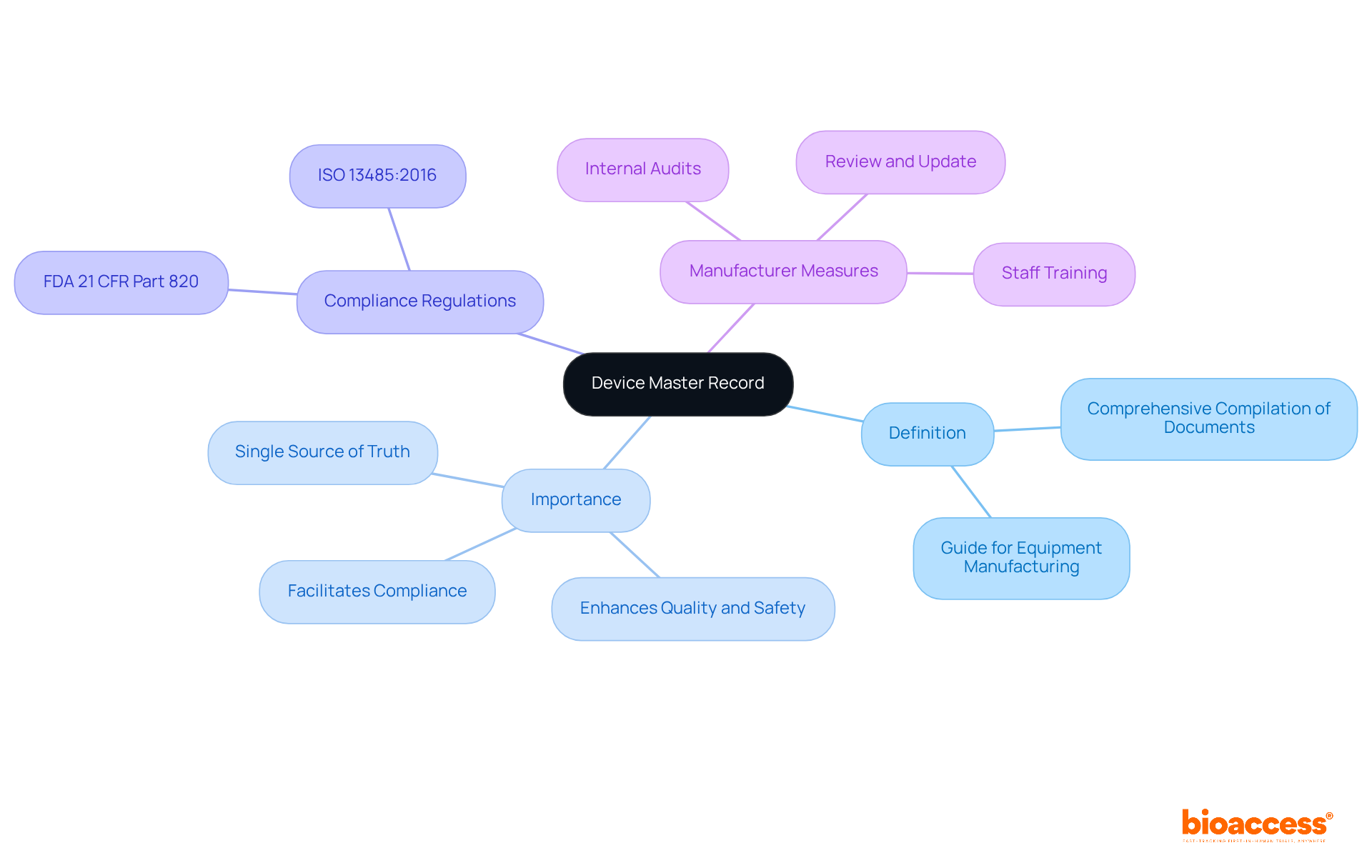
A master device record (MDR) serves as a comprehensive compilation of documents that delineate the procedures, specifications, and quality assurance measures essential for the production of a medical instrument. It functions as the definitive guide for all facets of equipment manufacturing, ensuring that each component adheres to legal standards.
The importance of a master device record (DMR) lies in its ability to provide a structured approach to documentation, which is crucial for compliance with regulations such as FDA 21 CFR Part 820 and ISO 13485:2016. Experts like Ana Criado, Director of Compliance and a professor in biomedical engineering, assert that a well-maintained master device record not only facilitates compliance but also enhances the overall quality and safety of medical instruments. Her extensive experience in governance, particularly regarding Colombian laws, underscores the necessity of a robust DMR in navigating complex compliance landscapes.
Additionally, Katherine Ruiz, a compliance specialist for medical devices and in vitro diagnostics, highlights the importance of integrating local compliance insights into the master device record practices.
To ensure adherence, manufacturers must:
These measures not only streamline production but also equip companies for effective responses during regulatory audits.
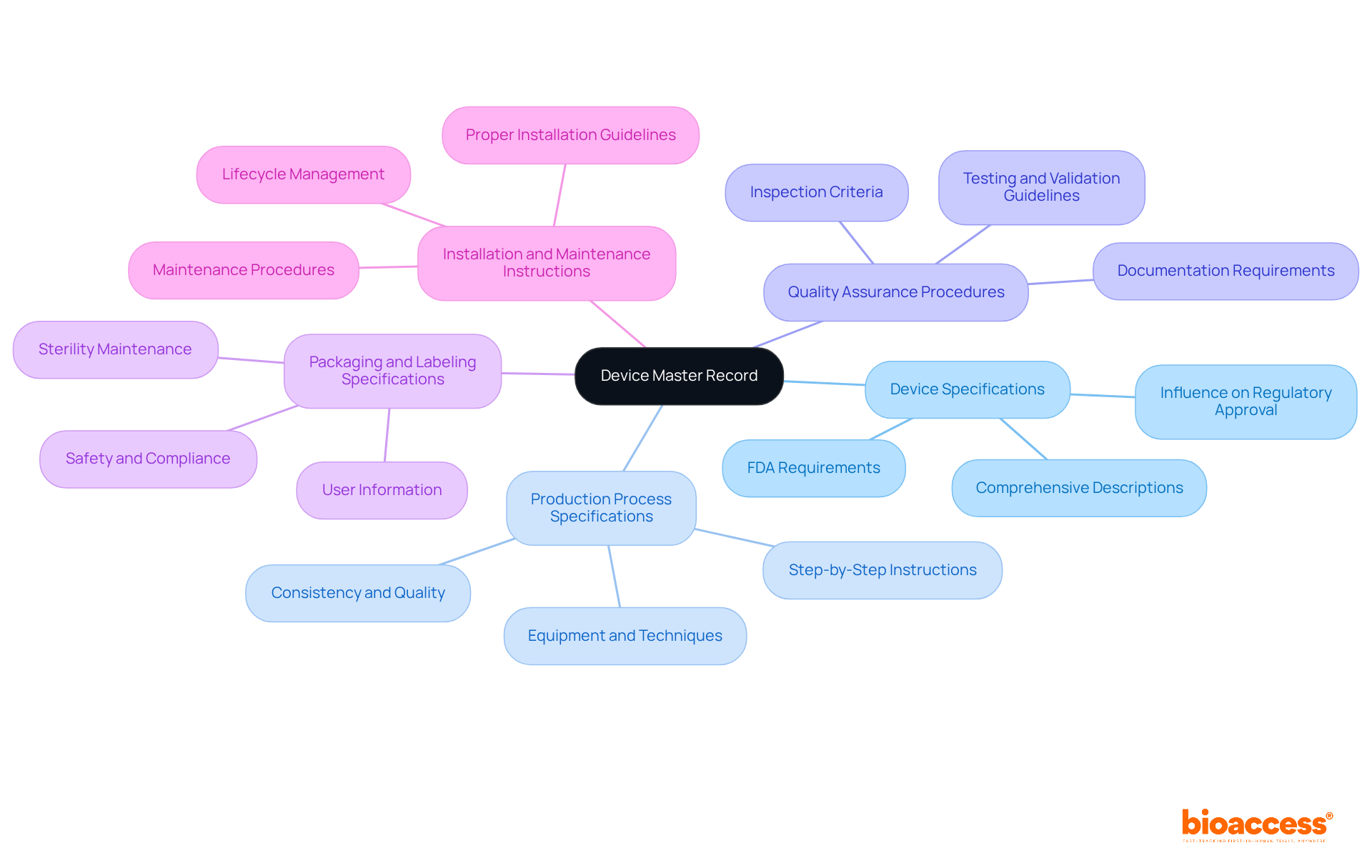
Key elements of a master device record are essential for ensuring compliance and facilitating successful medical product development. Understanding these elements is crucial for every Director in the field.
Device Specifications: This includes comprehensive descriptions of the device's design, materials, and intended use. Detailed specifications are vital, as they significantly influence regulatory approval procedures, ensuring that products meet safety and efficacy standards. According to the FDA, the DMR must include specifications for the apparatus such as drawings and component details, as outlined in 21 CFR Part 820.181.
Production Process Specifications: Step-by-step instructions on how the product is manufactured are included here. This section outlines the equipment and techniques used, which are essential for maintaining consistency and quality in production. A clearly defined production process reduces variations and defects, resulting in higher quality products. The FDA emphasizes that manufacturers must provide specifications for production processes to ensure reliable manufacturing.
Quality Assurance Procedures: Guidelines for testing and validating the apparatus are outlined to guarantee adherence to safety and efficacy standards. Quality assurance procedures must specify testing methods, inspection criteria, and documentation requirements, which are essential for regulatory adherence and maintaining product integrity. As noted by Joe Byrne, CEO of Cognidox, maintaining a DMR is essential for effective manufacturing data management.
Packaging and Labeling Specifications: This section outlines how the item should be packaged and labeled to ensure safety and compliance during distribution. Proper packaging is essential for maintaining sterility and preventing damage, while accurate labeling provides necessary information for users. The FDA mandates that packaging and labeling specifications must be included in the master device record to ensure safe utilization.
Installation and Maintenance Instructions: Directions for proper installation and maintenance are crucial for ensuring the apparatus functions as intended throughout its lifecycle. Clear instructions help prevent errors and accidents, ensuring safe and effective use of the equipment. Guidelines for installation and maintenance must be documented in the master device record to support the lifecycle management of the equipment.
Integrating these components into the master device record not only aids in meeting standards but also improves the overall quality and dependability of medical products, ultimately resulting in safer and more effective offerings in the market. For instance, a well-maintained DMR can significantly simplify the approval process for new equipment, as demonstrated in case studies such as the DMR Index for Cardiac Pacemakers.
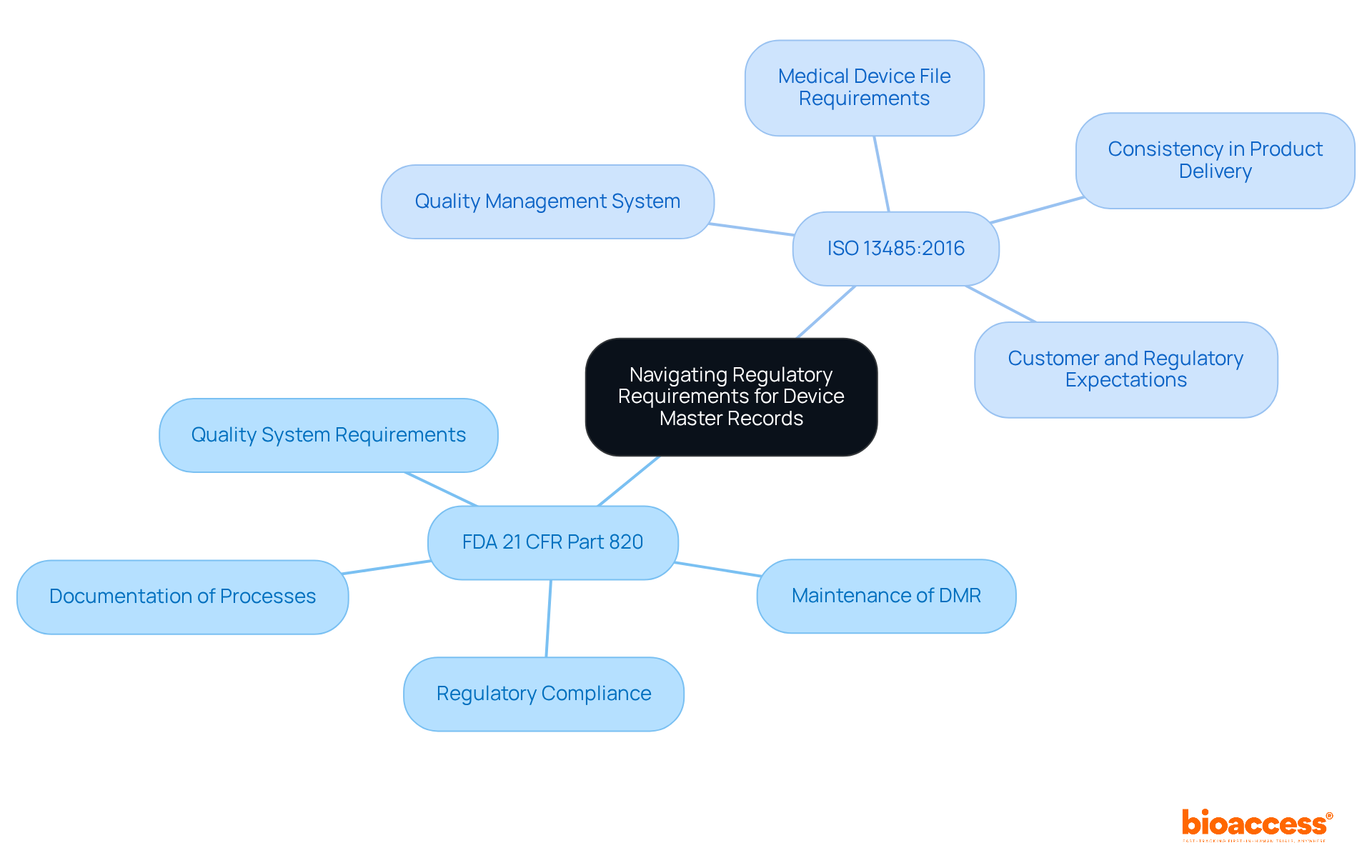
Navigating regulatory requirements for Device Master Records (DMRs) necessitates a thorough understanding of the guidelines established by regulatory bodies such as the FDA and ISO. Key regulations include:
FDA 21 CFR Part 820: This regulation delineates the quality system requirements for medical device manufacturers, mandating the maintenance of a DMR. It guarantees that producers record all processes and specifications associated with the product, which is essential for adherence and market authorization.
ISO 13485:2016: This global standard specifies the criteria for a quality management system, necessitating organizations to show their ability to consistently deliver medical products and associated services that fulfill customer and regulatory expectations. Under section 4.2.3, it necessitates a Medical Device File, which serves a similar purpose to the DMR.
Organizations must ensure that their DMRs are routinely updated to reflect any changes in regulations or equipment specifications. This includes documenting modifications in design, production processes, and quality assurance procedures. Frequent updates are crucial not only for adherence but also for preserving the integrity and safety of medical equipment throughout their lifecycle.
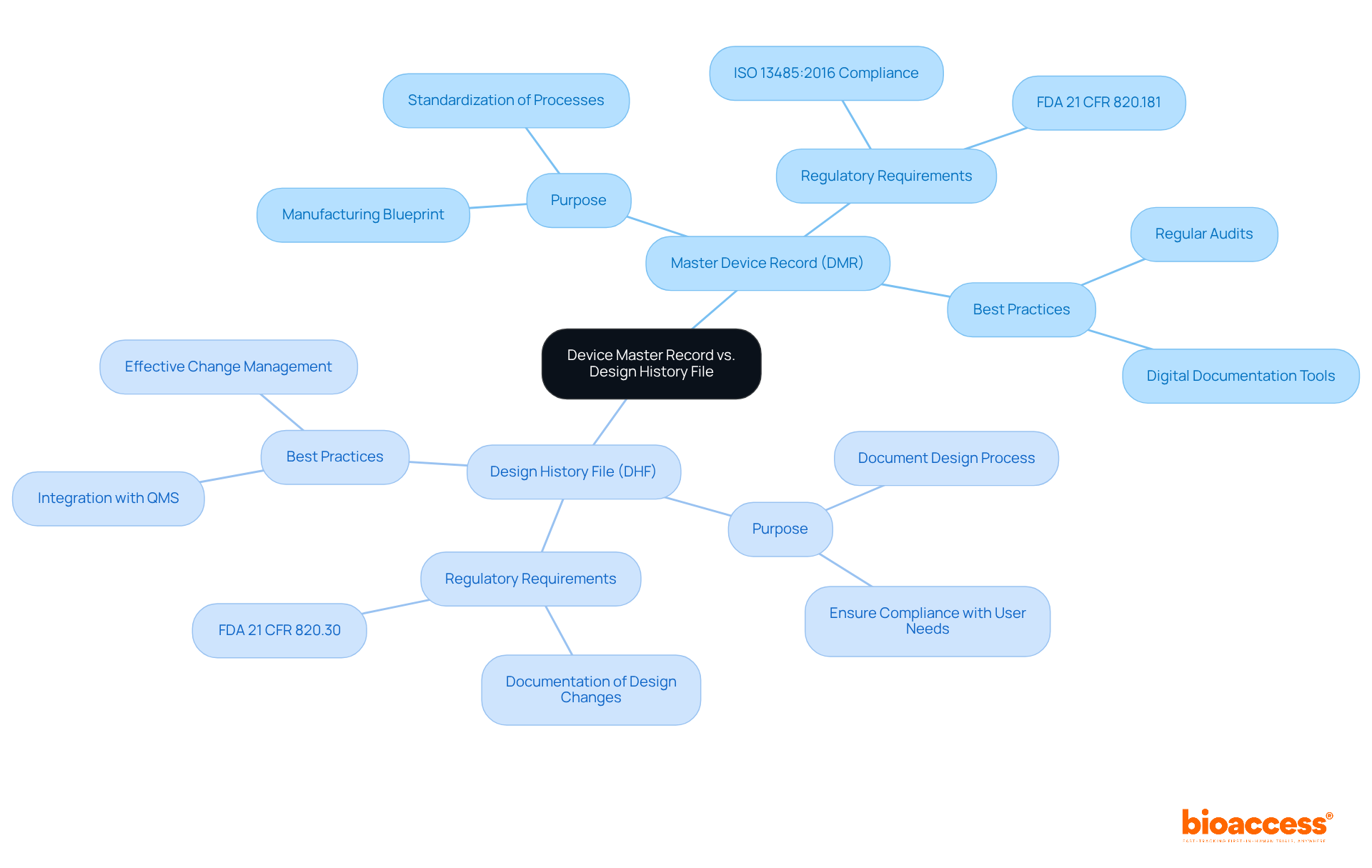
The master device record (MDR) and Design History File (DHF) are integral components of the medical device lifecycle, each serving a unique purpose. The DMR serves as a comprehensive blueprint for manufacturing a medical device, encompassing all specifications, production processes, quality assurance protocols, and packaging requirements. Maintaining an up-to-date master device record is essential; it ensures that all production processes are standardized and compliant with regulatory standards, ultimately leading to consistent product quality. According to FDA regulations (21 CFR 820.181), each manufacturer must maintain a master device record for products and ensure that it is prepared and approved in accordance with established guidelines.
The DHF documents the complete design and development process of a medical instrument. It contains essential documents that illustrate adherence to the approved design plan, ensuring that the apparatus meets user needs and regulatory standards. The DHF is crucial for recording design inputs, outputs, and modifications, which are important for ensuring compliance throughout the product's lifecycle. The FDA requires that each producer create and uphold a DHF for every kind of product (21 CFR 820.30).
Precise upkeep of both the master device record and the DHF is not merely a compliance necessity; it is essential for guaranteeing the safety and efficacy of medical instruments. Research shows that organizations with strong documentation practices encounter fewer regulatory issues and improved product quality. For instance, a case study titled 'Managing DHF with Medical Product Quality Systems' demonstrates how efficient handling of these documents is vital for compliance with regulations and the safety and efficacy of medical products.
Companies that implement best practices for managing their master device record and DHF—such as regular audits and utilizing digital documentation tools—significantly reduce the risk of non-compliance and improve overall efficiency. As Desiree Tarranco, a Quality Assurance Professional, states, "QMS software is crucial for overseeing the requirements related to design history files, master records, and history records in the medical equipment industry." As the medical equipment industry evolves, understanding and maintaining these records accurately will be crucial for navigating regulatory landscapes and ensuring successful product outcomes.
To improve adherence and efficiency, manufacturers should consider conducting regular audits and using digital tools for document management.
Documentation is fundamental in managing the master device record, ensuring that every aspect of a device's design, production, and quality control is meticulously recorded and readily accessible. Key best practices for effective DMR documentation management include:
A master device record (DMR) can be stored digitally and is typically managed on an electronic quality management system, which enhances the efficiency of documentation management. Efficient documentation management not only aids adherence to FDA regulations but also greatly improves the overall quality and reliability of medical devices. As industry specialists highlight, preserving a single version of the truth through structured documentation is essential for successful product development and compliance audits. To further improve DMR management, companies should consider utilizing automated solutions, which can accelerate new product introductions and enhance collaboration with partners and suppliers.

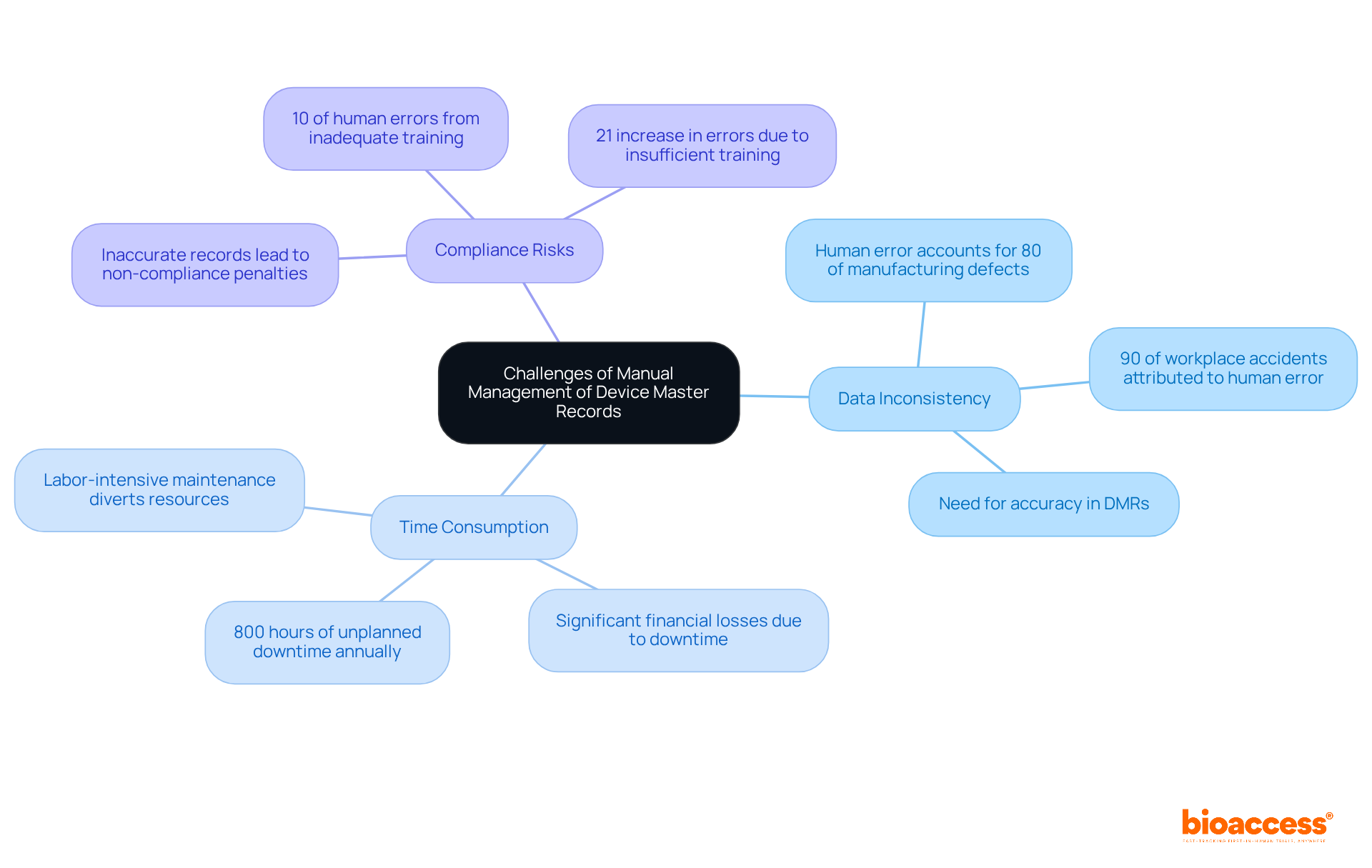
Manual management of Device Master Records (DMRs) poses significant challenges that can jeopardize adherence and operational efficiency. Notable issues include:
In light of these challenges, organizations must recognize the urgency of adopting automated solutions to streamline DMR management, thus enhancing accuracy and efficiency. Automating routine tasks can significantly reduce human errors, representing a vital step towards bolstering operational integrity.
Leveraging automation for Device Master Record (DMR) management significantly enhances how organizations manage their documentation. The benefits of automation are substantial:
Moreover, approximately 60% of all jobs have at least 30% of their activities that can be automated, highlighting the potential for automation to enhance job efficiency within the Medtech industry. Technology leaders emphasize the importance of automation in improving operational efficiency. For instance, Bill Gates noted that automation applied to efficient operations magnifies that efficiency, while poorly run operations may exacerbate inefficiencies. By adopting automated solutions, organizations can enhance their DMR management processes, ultimately focusing on innovation and improving patient outcomes. Successful implementations of automated DMR systems in Medtech have demonstrated that these tools not only streamline workflows but also foster a culture of compliance and quality assurance.

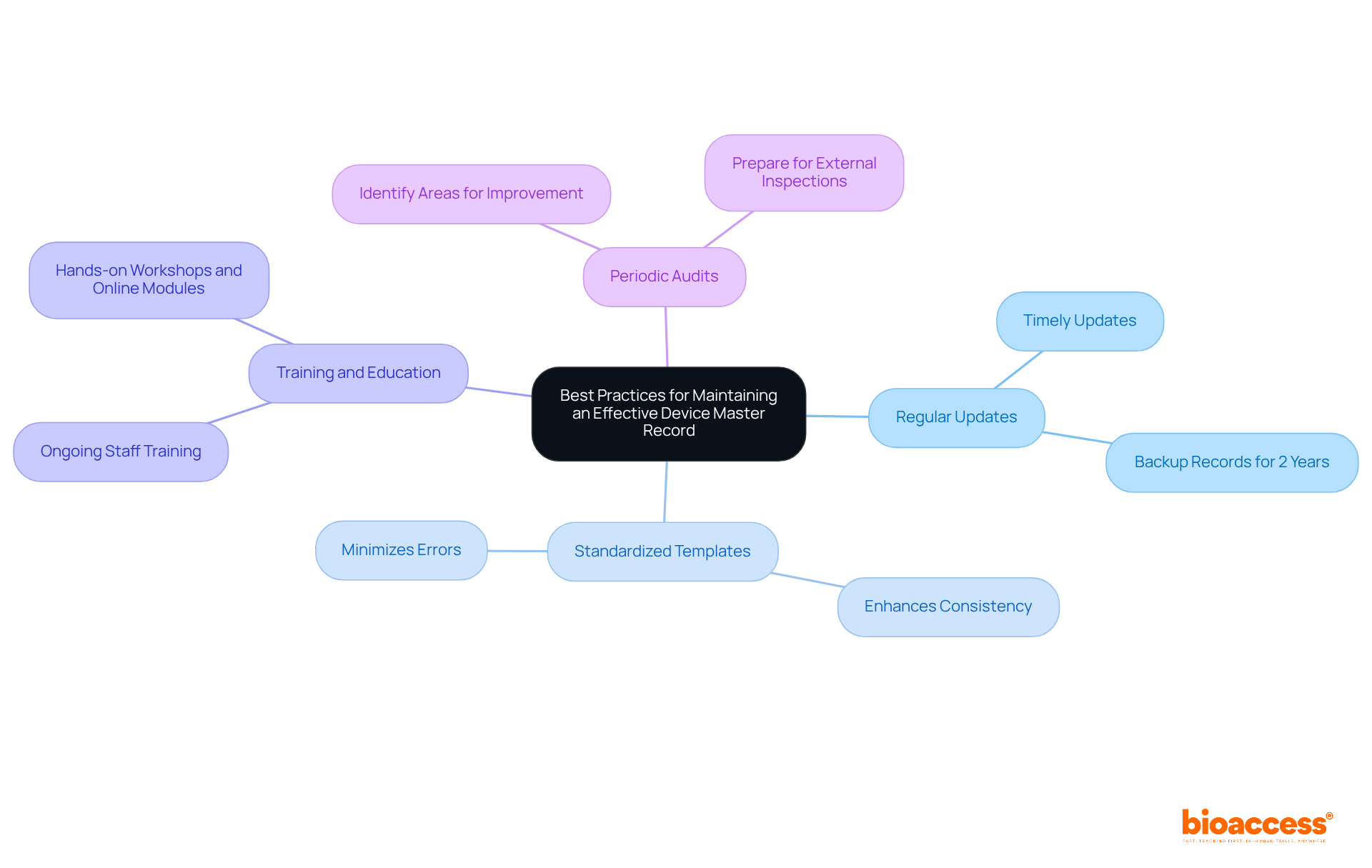
Best practices for maintaining an effective master device record are critical for ensuring compliance and operational efficiency in clinical research.
Regular Updates: Timely updates to the DMR are essential to reflect any changes in design, production processes, or regulatory requirements. This practice ensures that the master device record remains a reliable source of truth throughout the product lifecycle. Records must be backed up and kept for at least 2 years to meet compliance standards.
Standardized Templates: Utilizing standardized templates for documentation enhances consistency and minimizes errors. This approach streamlines the documentation process, making it easier to manage and audit records.
Training and Education: Ongoing training for staff involved in DMR management is crucial. Regular training sessions ensure that personnel are well-versed in best practices and stay informed about regulatory changes. Effective training programs, such as those that incorporate hands-on workshops and online modules, can significantly influence adherence. As Jesseca Lyons states, "Auditors and inspectors will review your DHF to confirm adherence, so maintaining your design history files well organized in an industry-specific medical device QMS is crucial."
Periodic Audits: Conducting regular audits of the master device record is essential for identifying areas for improvement and ensuring adherence to standards. These audits help maintain the integrity of the master device record and prepare organizations for external inspections. With the upcoming transition to the Quality Management System Regulation (QMSR) on February 2, 2026, it is more important than ever to ensure that the master device record (DMR) is compliant with evolving regulations.
By adhering to these best practices, organizations can maintain high-quality master device records that not only support successful clinical research outcomes but also enhance overall operational efficiency.
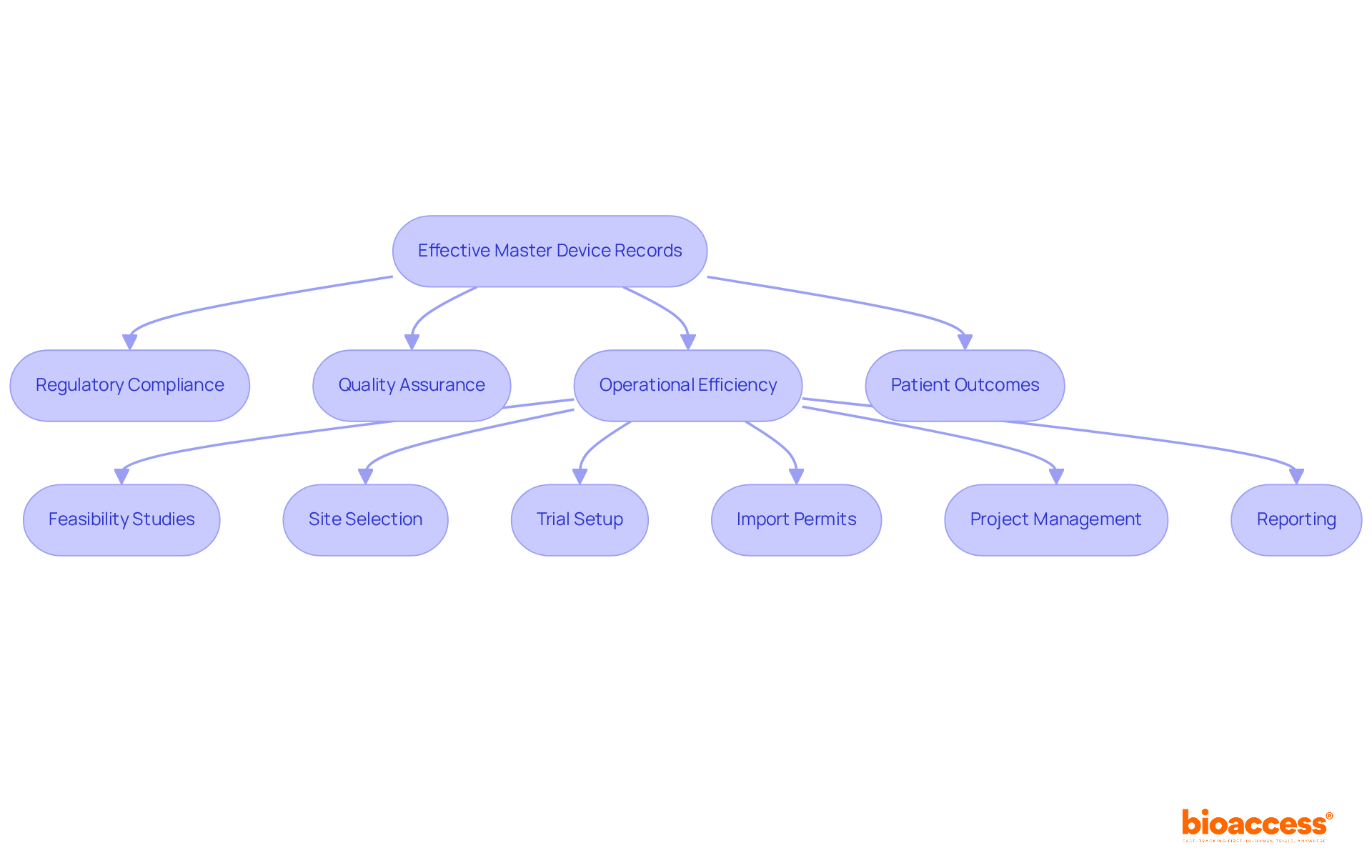
Effective master device records (MDRs) have a profound impact on the success of clinical research. They ensure that regulatory compliance is maintained, facilitating adherence to essential requirements and reducing the risk of delays or penalties. This is particularly crucial in the context of clinical trials, where compliance with local regulations, such as those enforced by INVIMA in Colombia, is vital for successful study execution.
Moreover, comprehensive documentation supports quality assurance processes, ensuring that products meet safety and efficacy standards. This commitment is reinforced by bioaccess's dedication to reviewing and providing feedback on study documents, ensuring compliance with country-specific requirements.
Additionally, well-maintained DMRs enhance operational efficiency, allowing for faster project timelines and improved resource allocation. The comprehensive clinical trial management services offered by bioaccess, including:
significantly contribute to this efficiency.
Ultimately, effective management of the master device record, supported by robust clinical trial services, is pivotal for the successful development and commercialization of medical devices. This not only benefits manufacturers but also enhances patient outcomes, underscoring the importance of collaboration in the Medtech landscape.
Effective management of master device records (MDRs) is pivotal for the success of clinical research and the overall quality of medical devices. By centralizing critical documentation, organizations can streamline compliance with regulatory standards, enhance operational efficiency, and ultimately improve patient outcomes. The insights provided throughout this article highlight the importance of a well-structured MDR in navigating the complexities of the Medtech landscape.
Key components of a master device record, including device specifications, production processes, and quality assurance procedures, play a crucial role in ensuring adherence to regulations such as FDA 21 CFR Part 820 and ISO 13485:2016. The integration of best practices—such as regular updates, standardized templates, and thorough training—further strengthens the integrity of these records. Additionally, leveraging automation can significantly reduce errors and enhance compliance, making it an essential strategy for modern Medtech companies.
The significance of maintaining effective device master records cannot be overstated. As the industry evolves, so do the challenges and regulatory requirements. Organizations must prioritize robust documentation practices and consider adopting automated solutions to stay ahead. By doing so, they not only foster a culture of compliance but also pave the way for innovation and improved patient care in the ever-changing landscape of medical technology.
What is the purpose of a master device record (MDR) in Medtech?
A master device record serves as a comprehensive compilation of documents that outline the procedures, specifications, and quality assurance measures necessary for the production of a medical instrument, ensuring compliance with legal standards.
How does bioaccess® contribute to clinical research in Latin America?
bioaccess® accelerates clinical research by centralizing and organizing crucial documentation, enhancing approval processes, and significantly reducing the time to market for innovative medical products.
What types of clinical trials does bioaccess® manage?
bioaccess® specializes in managing early-feasibility studies, first-in-human studies, and post-market clinical follow-up studies.
What are the benefits of maintaining a well-organized master device record?
A well-maintained master device record streamlines the approval process, ensures compliance with regulations, enhances patient outcomes, and fosters advancements in medical knowledge.
What are the key components of a master device record that every director should know?
Key components include device specifications, production process specifications, quality assurance procedures, packaging and labeling specifications, and installation and maintenance instructions.
Why is it important to regularly review and update the master device record?
Regular reviews and updates ensure compliance with regulations, enhance the quality and safety of medical instruments, and prepare manufacturers for effective responses during regulatory audits.
How can a master device record affect the FDA submission process?
A well-maintained master device record can streamline the FDA submission process, reducing the average time for submissions and minimizing requests for additional information that can delay approvals.
What are the financial benefits of using bioaccess® for clinical trials?
bioaccess® achieves patient enrollment 50% faster and saves $25K per patient by providing FDA-ready data.
What regulations must manufacturers comply with regarding master device records?
Manufacturers must comply with regulations such as FDA 21 CFR Part 820 and ISO 13485:2016 regarding the documentation and maintenance of master device records.
What role do local compliance insights play in the master device record practices?
Integrating local compliance insights into master device record practices is essential for navigating complex compliance landscapes and ensuring adherence to specific regulatory requirements.