


The stopcock valve 3 way stands as a pivotal element in contemporary medical procedures, empowering clinicians to manage fluid flow with both precision and efficiency. In an era where healthcare is rapidly evolving, grasping the benefits and best practices associated with this device is vital for enhancing patient care and safety. Yet, with the growing complexity of medical technologies, how can clinicians ensure they are effectively utilizing these valves while minimizing risks? This article explores seven essential insights that underscore the significance of the stopcock valve 3 way, providing invaluable guidance for healthcare professionals as they navigate its application in clinical settings.
bioaccess® excels in expediting clinical research for medical devices, leveraging its strategic presence in Latin America, particularly Colombia. This region offers significant advantages, including over 30% cost savings compared to North America and Western Europe, along with a regulatory review process that takes only 90-120 days. Such agility is crucial for the swift introduction of innovative medical technologies, particularly for devices like the stopcock valve 3 way, which are vital for enhancing care and treatment efficiency.
Industry leaders emphasize that regulatory speed directly correlates with clinical trial success rates. This underscores the importance of rapid approvals in the competitive medical device landscape. With a steadfast commitment to high-quality clinical research, bioaccess® ensures that new devices undergo rigorous evaluation, ultimately leading to safer and more effective medical solutions.
Furthermore, Colombia's robust healthcare system, ranked among the best globally, along with its universal healthcare coverage, facilitates efficient participant recruitment. Recent developments in 2025 highlight bioaccess®'s ongoing efforts to support manufacturers in navigating complex regulatory environments, further solidifying its role as a key player in the advancement of medical technology. Collaboration is essential in this landscape, and bioaccess® stands ready to guide partners through the challenges of clinical research.
The stopcock valve 3 way mechanism serves as a pivotal instrument in medical procedures, enabling precise regulation of liquid movement through three distinct ports. This functionality not only allows healthcare providers to administer various medications or solutions simultaneously but also significantly enhances care efficiency. By facilitating swift transitions between different infusion lines without the need to detach the patient, this device minimizes infection risks and ensures uninterrupted treatment.
Its versatility is particularly vital in intravenous (IV) therapy, where it streamlines the management of liquids and reduces the necessity for repeated vein punctures, thereby improving patient comfort and safety. Modern 3-way control devices incorporate advanced sealing systems that effectively mitigate liquid leakage, further bolstering safety during procedures. Clinicians recognize the critical role of the stopcock valve 3 way devices in maintaining hemodynamic stability and improving treatment outcomes, as evidenced by their widespread use in critical care settings and complex procedures like blood transfusions and cardiac catheterizations.
Real-world applications illustrate that employing these valves not only boosts fluid management efficiency but also enhances patient experiences by alleviating anxiety linked to multiple needle sticks. Furthermore, the integration of innovations such as anti-reflux mechanisms and built-in filters can further elevate patient safety and procedural efficiency.
The Dispoway 3-way valve stands out with its innovative design, significantly enhancing usability in clinical environments. Made from lipid-resistant materials, it effectively prevents micro-cracking, ensuring durability even when used with lipid-based medications. This is crucial, as conventional valves often face contamination issues due to liquid retention in their design. The low dead space design of the Dispoway valve minimizes this risk, contributing to improved hygiene and patient safety.
Moreover, the handle's smooth rotation allows for precise control over fluid flow, making it easier to manage multiple infusions. These advancements not only boost treatment efficacy but also meet the increasing demand for safer medical devices. Recent case studies reveal that lipid-resistant valves have led to a notable decrease in contamination rates compared to traditional options, with some studies indicating a reduction of up to 30%. Healthcare experts emphasize that incorporating lipid-resistant materials in control devices is vital for reducing infection risks and enhancing overall patient outcomes.
Additionally, the advanced medical valve market is projected to grow at a CAGR of 8.20% from 2025 to 2032, underscoring the rising demand for innovations like the Dispoway valve. As clinical research continues to evolve, the importance of such advancements cannot be overstated.

To ensure the safe and effective use of the 3-way valve, clinicians must adhere to several best practices. Priming the valve before use is crucial; it eliminates air bubbles that can lead to air embolisms, a serious complication in intravenous therapy. Statistics reveal that improper valve use is linked to a significant number of air embolism incidents, underscoring the importance of this initial step.
Next, verifying the placement of the stopcock valve 3 way handle is essential to ensure the proper flow direction before administering liquids. Additionally, clinicians should avoid over-rotating the handle beyond 180 degrees to prevent damage to the valve. This caution is vital, as the rotating handle provides real-time control over liquid pathways, ensuring accurate infusion rates.
Regular inspection and maintenance of the valve are also critical to ensure its functionality and prevent contamination. Case studies have shown that neglecting the maintenance of the stopcock valve 3 way can result in serious complications, including fluid leakage and air embolisms. By following these guidelines, healthcare professionals can significantly reduce the risk of air embolisms and enhance safety during IV therapy. Clinicians are encouraged to regularly review and apply these best practices to achieve optimal results for their patients.
Lipid resistance in control devices is essential for maintaining their integrity when interacting with lipid-based medications. Traditional materials often degrade upon exposure to lipids, leading to micro-cracking and potential leakage incidents. For instance, studies have shown that non-lipid resistant connectors can cause significant leakage, jeopardizing both safety and treatment effectiveness.
In contrast, the stopcock valve 3 way is designed with lipid-resistant materials that effectively withstand these challenges, ensuring reliable performance during infusions. This compatibility is crucial not only for preventing contamination but also for preserving the effectiveness of medications throughout the treatment process. Industry leaders emphasize that material compatibility is vital in the design of valve systems, as it directly impacts user outcomes and the overall safety of infusion devices.
By utilizing lipid-resistant materials, clinicians can significantly enhance the reliability of their infusion systems, ultimately improving patient care. This proactive approach not only safeguards against potential complications but also reinforces the commitment to delivering high-quality healthcare.
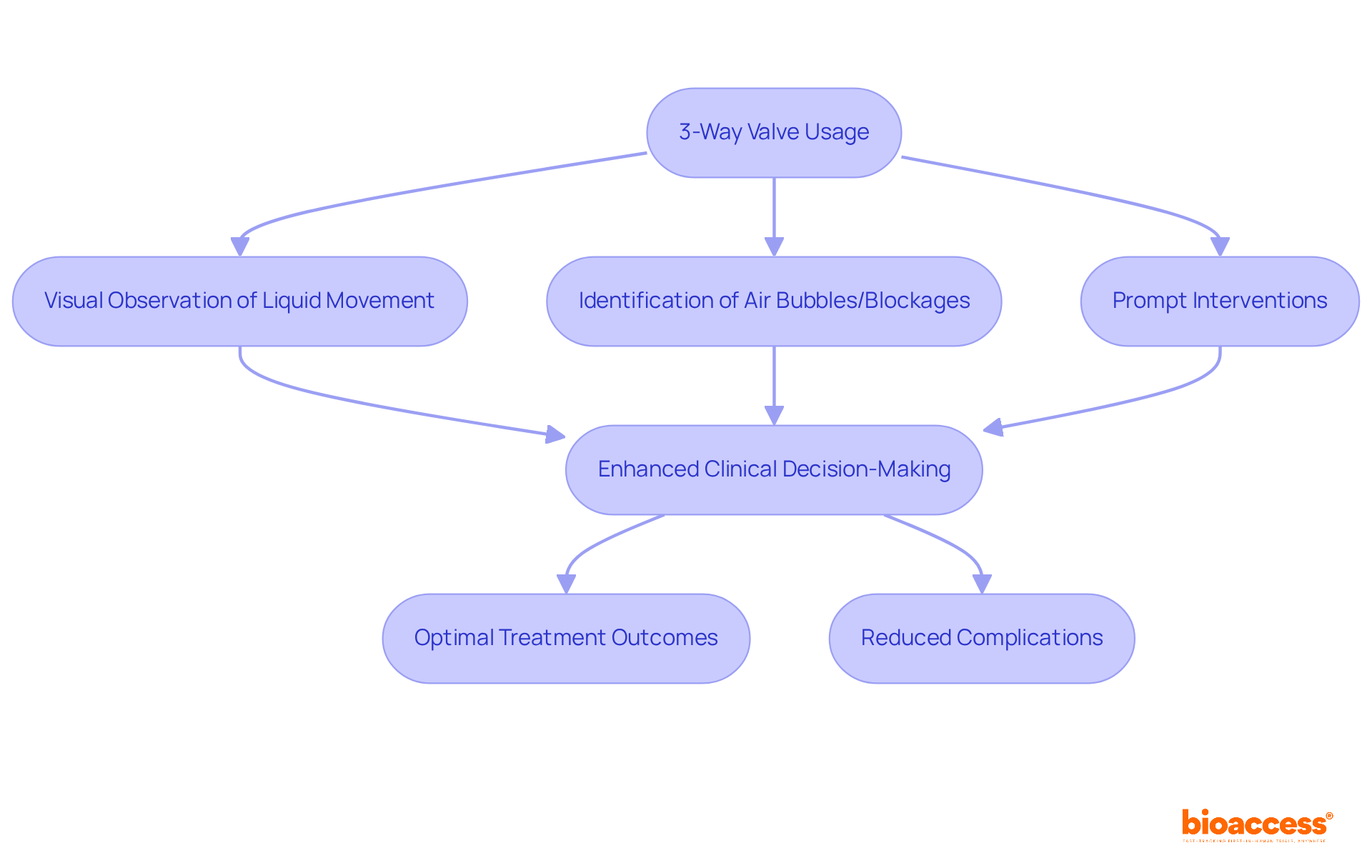
The clear flow passage in the 3-way valve is a crucial characteristic that allows clinicians to visually observe liquid movement during medical procedures. This capability is vital for identifying air bubbles, blockages, or variations in liquid dynamics, enabling prompt interventions. Enhanced visibility supports better clinical decision-making, as clinicians can quickly assess infusion statuses and make necessary adjustments, ultimately ensuring optimal treatment outcomes.
Research has shown that systems utilizing a stopcock valve 3 way significantly reduce the likelihood of complications related to air embolisms, thereby enhancing safety for patients. By facilitating real-time monitoring of fluid flow, these devices contribute to a safer clinical environment, underscoring the importance of visibility in effective fluid management.
Furthermore, the global Intravenous (IV) Fluid Monitoring Devices Market is projected to grow at a rate of 6.6% CAGR from 2024 to 2030, driven by an increasing focus on safety and clinical outcomes in healthcare delivery. This trend highlights the essential role of clear control mechanisms in modern medical practices.
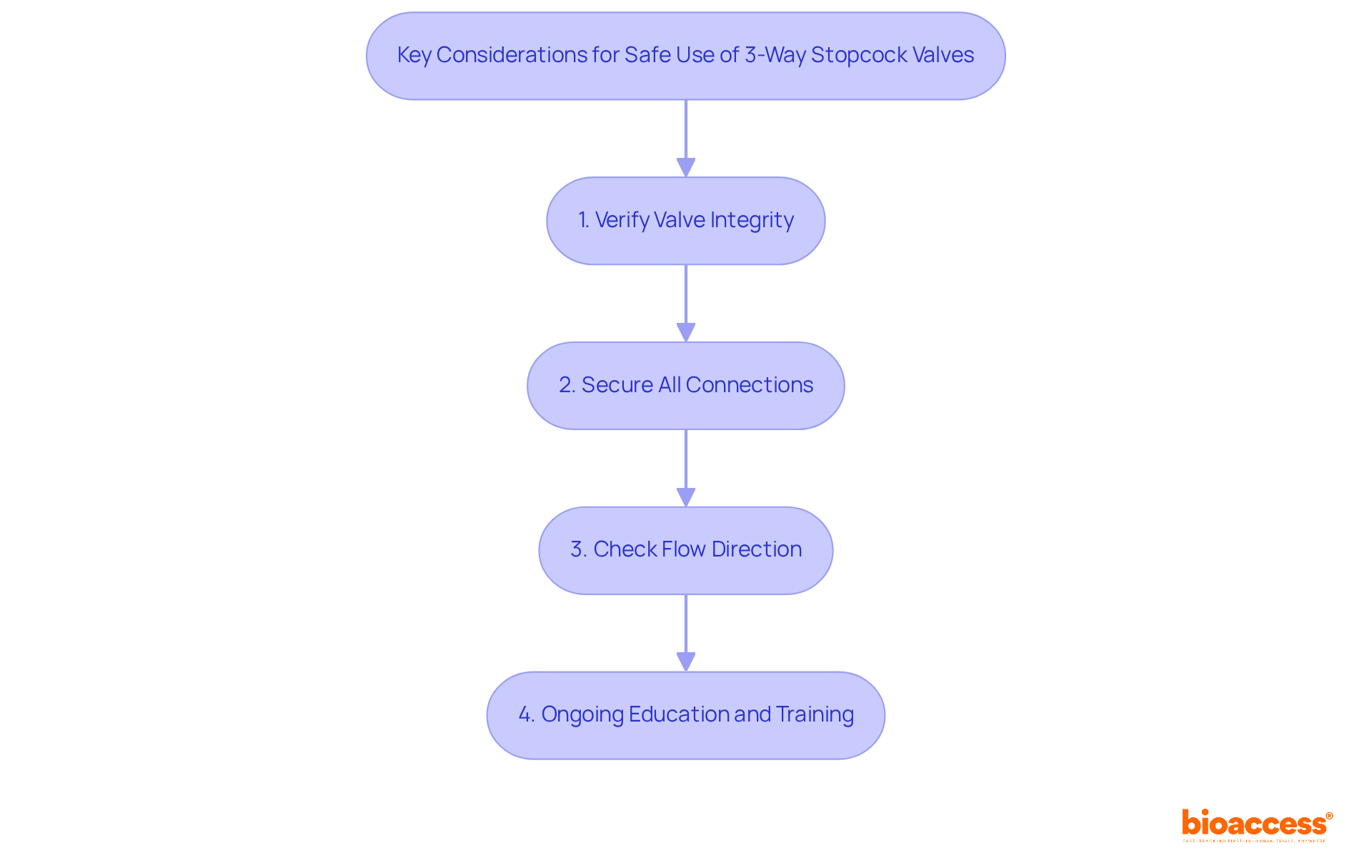
Clinicians must prioritize several essential factors when using the stopcock valve 3 way to ensure both patient safety and procedural effectiveness. First, verifying the integrity of the valve prior to use is crucial. Inspecting for any signs of wear or damage that could compromise functionality is essential. Routine inspections for loosening or shifting joints are vital, particularly when several valves are linked, as this can lead to blood and fluid leakage. Research indicates that improper management of control devices can significantly elevate infection rates, underscoring the necessity for caution in preserving sterile settings. Healthcare professionals emphasize that maintaining sterility during handling is paramount to minimizing infection risks.
Second, securing all connections is critical to prevent leaks and contamination. A systematic review has highlighted that incorrect management of control devices can greatly increase infection rates, reinforcing the need for vigilance in upholding sterile environments. Maintaining sterility during handling remains a top priority for healthcare professionals to mitigate infection risks.
Third, clinicians should always be aware of the flow direction indicated by the handle to avoid administering fluids incorrectly, which can have serious implications for safety. Lastly, ongoing education and training on the proper use of the stopcock valve 3 way are essential. These efforts help reinforce best practices and enhance overall patient care. By adhering to these considerations, clinicians can significantly improve the safety and effectiveness of their procedures.
The significance of the 3-way stopcock valve in clinical settings is paramount, as it plays a crucial role in enhancing patient care through improved efficiency and safety. This device not only facilitates the simultaneous administration of multiple medications but also minimizes infection risks, thereby enhancing the overall treatment experience for patients.
Key insights from the article underscore the advantages of using the stopcock valve 3-way, including innovative designs like the Dispoway valve, which offers lipid resistance and enhanced usability. Best practices for clinicians highlight the importance of proper handling and maintenance to prevent complications such as air embolisms and infections. Furthermore, the transparent flow channel feature significantly aids in monitoring fluid dynamics, ensuring timely interventions and better clinical outcomes.
As the healthcare landscape evolves, embracing these advancements and adhering to best practices becomes essential for clinicians. By prioritizing the safe and effective use of the 3-way stopcock valve, healthcare professionals can significantly improve patient safety and treatment efficacy. The commitment to innovation and excellence in clinical practices ultimately reinforces the overarching goal of delivering high-quality healthcare.
What is bioaccess® and what role does it play in clinical research for medical devices?
bioaccess® is a company that specializes in expediting clinical research for medical devices, particularly in Latin America, with a strong presence in Colombia. It helps manufacturers navigate regulatory environments and ensures rigorous evaluation of new devices to enhance their safety and effectiveness.
What are the advantages of conducting clinical research in Colombia through bioaccess®?
Conducting clinical research in Colombia via bioaccess® offers over 30% cost savings compared to North America and Western Europe. Additionally, the regulatory review process in Colombia takes only 90-120 days, facilitating quicker introduction of medical technologies.
How does regulatory speed affect clinical trial success rates?
Industry leaders emphasize that the speed of regulatory approvals is directly correlated with clinical trial success rates, highlighting the importance of rapid approvals in the competitive medical device landscape.
What is the significance of the stopcock valve 3 way in medical procedures?
The stopcock valve 3 way is crucial for regulating liquid movement through three ports, allowing healthcare providers to administer multiple medications or solutions simultaneously, enhancing care efficiency and minimizing infection risks.
In what ways does the stopcock valve 3 way improve patient safety and comfort?
The stopcock valve 3 way improves patient safety by streamlining IV therapy management, reducing the need for repeated vein punctures, and incorporating advanced sealing systems to mitigate liquid leakage. This enhances patient comfort by minimizing anxiety related to multiple needle sticks.
What innovations are being integrated into modern 3-way stopcock valves?
Modern 3-way stopcock valves are incorporating innovations such as anti-reflux mechanisms and built-in filters, which further enhance patient safety and procedural efficiency.
How does bioaccess® support manufacturers in 2025?
In 2025, bioaccess® continues to support manufacturers by helping them navigate complex regulatory environments, thereby solidifying its role in advancing medical technology and facilitating efficient participant recruitment due to Colombia's robust healthcare system.