


The article titled "7 Essential IVD Insights for Clinical Research Directors" provides critical insights crucial for Clinical Research Directors navigating the complexities of in vitro diagnostics (IVD) regulations and processes. It underscores the necessity of:
These elements are essential for ensuring successful clinical trials and facilitating timely market entry for innovative diagnostic devices.
The landscape of in vitro diagnostics (IVD) is rapidly evolving, presenting both opportunities and challenges for Clinical Research Directors. With the introduction of stringent regulations and classification systems, understanding the nuances of IVD compliance has never been more critical. This article delves into essential insights that can empower directors to navigate these complexities effectively, ensuring that their clinical research not only meets regulatory standards but also accelerates the development of innovative diagnostic solutions.
How can directors leverage these insights to enhance trial efficiency and patient safety amidst the changing regulatory environment?
bioaccess® empowers Medtech startups by leveraging its expertise in early-stage trials, particularly in Colombia, which stands as a premier location for first-in-human assessments. With significant cost reductions exceeding 30% compared to North America and Western Europe, alongside a regulatory review process that spans just 90-120 days, bioaccess® ensures that startups can adeptly navigate the complexities of clinical trials.
Colombia boasts a high-quality healthcare system, consistently ranked among the best in the world, providing access to a diverse patient population of over 50 million, with 95% covered by universal healthcare. Furthermore, hospitals in Colombia are authorized to conduct research involving pharmaceutical drugs only after achieving stringent ICH/GCP certification, thereby upholding rigorous quality standards.
The country also presents appealing R&D tax incentives, including substantial tax deductions and grants for innovative projects. This combination of advantages enables bioaccess® to facilitate the completion of trials within 4-6 weeks, significantly accelerating product development timelines and ensuring that startups can effectively compete in the dynamic Medtech landscape.

Navigating the intricate landscape of IVD regulations is essential for Clinical Research Directors, particularly in light of the latest FDA guidelines for 2025. Compliance with these regulations is not merely a formality; it is a critical factor that ensures diagnostic devices meet stringent safety and efficacy standards before market entry. The IVDR, or In Vitro Diagnostic Regulation, which came into effect on May 26, 2022, introduces a risk-based classification system that categorizes devices into four classes (A, B, C, and D), significantly impacting the conformity assessment process and the level of scrutiny required for CE marking.
Key compliance requirements involve:
This shift emphasizes the importance of thorough initial submissions, as evidenced by the statistic that 67% of FDA 510(k) submissions resulted in requests for additional information during the substantive review process in the year leading up to September 2022. Such delays can have a cascading effect on study timelines and patient safety.
Successful navigation of FDA IVD guidelines is exemplified by organizations that have implemented stringent validation processes for their IVD assays, ensuring accuracy, precision, and compliance with IVDR standards. For example, bioaccess has successfully assisted Medtech and Biopharma innovators in obtaining approvals rapidly through thorough trial management services. These services encompass:
This proactive strategy for compliance can significantly improve efficiency in studies.
Specialist viewpoints highlight the importance of comprehending these governing structures, as they directly affect medical investigation timelines. With the medical device industry estimated to hit $530 billion in 2024, the impact of guidelines on research involving patients is more crucial than ever. By prioritizing compliance and staying informed about evolving regulations, Clinical Research Directors can mitigate risks and enhance the safety and efficacy of IVD. To maximize the benefits of these regulations, consider engaging with bioaccess to leverage their expertise in navigating the complexities of IVD.

In vitro diagnostics (IVD) are categorized into four risk classes—A, B, C, and D—based on their potential hazards. Class A represents low-risk devices, while Class D includes those that pose significant risks to patient health. Each classification involves specific compliance requirements that must be followed, making it essential for Clinical Research Directors to understand these distinctions. Comprehending IVD is crucial as its classifications directly impact study design, determine the type of data needed for regulatory approval, and influence the overall timeline for product commercialization.
Industry experts emphasize the importance of accurate classification. Emily Malmberg, a consultant in development, observes that 'many companies do not possess the evidence required by the IVDR,' highlighting the heightened scrutiny under the new regulations. The IVDR mandates a higher standard of medical evidence compared to its predecessor, the IVDD, complicating the approval process for many manufacturers in the IVD sector.
Current trends indicate a shift towards more stringent oversight requirements, with the IVDR fully in effect since May 2022. This regulation introduces a risk-based approach to classification, focusing on the consequences of inaccuracies rather than direct patient interaction. For instance, Class C IVDs, which include tests for cancer screening, present life-threatening risks if results are incorrect, necessitating rigorous compliance measures for IVDs.
The implications of these classifications extend to clinical study design. If an IVD has several intended uses, it is categorized in the highest risk class, greatly influencing the data collection strategy and approval process. Moreover, the categorization of accessories utilized with IVDs is handled independently, adding to the complexity of the oversight environment.
As the IVD market continues to evolve, comprehending these classifications and their oversight implications is crucial for Clinical Research Directors. By staying informed about the latest updates and trends, they can better navigate the complexities of IVD development and ensure successful market entry for their products. Additionally, utilizing perspectives from industry experts, including those presented by Steve Garchow, can offer useful tactics for tackling local compliance intricacies and enhancing trial frameworks in Latin America.

Obtaining an Investigational Device Exemption (IDE) is essential for conducting trials involving IVD devices. This process requires the submission of thorough documentation that outlines the device's intended use, study protocol, and supporting data demonstrating its safety and effectiveness. Clinical Research Directors must prioritize meticulous documentation to meet FDA standards, as this significantly impacts the approval timeline.
Recent years have seen the FDA streamline its processes; however, challenges persist. For instance, 67% of FDA 510(k) submissions received requests for additional information during the review cycle, underscoring the necessity for clarity and completeness in submissions. Moreover, recent changes in IDE submission requirements demand that Clinical Research Directors remain informed about evolving regulations to avoid delays and ensure compliance.
Engaging with regulatory specialists early in the process can provide valuable insights into these requirements, ultimately facilitating the approval process and expediting trials.

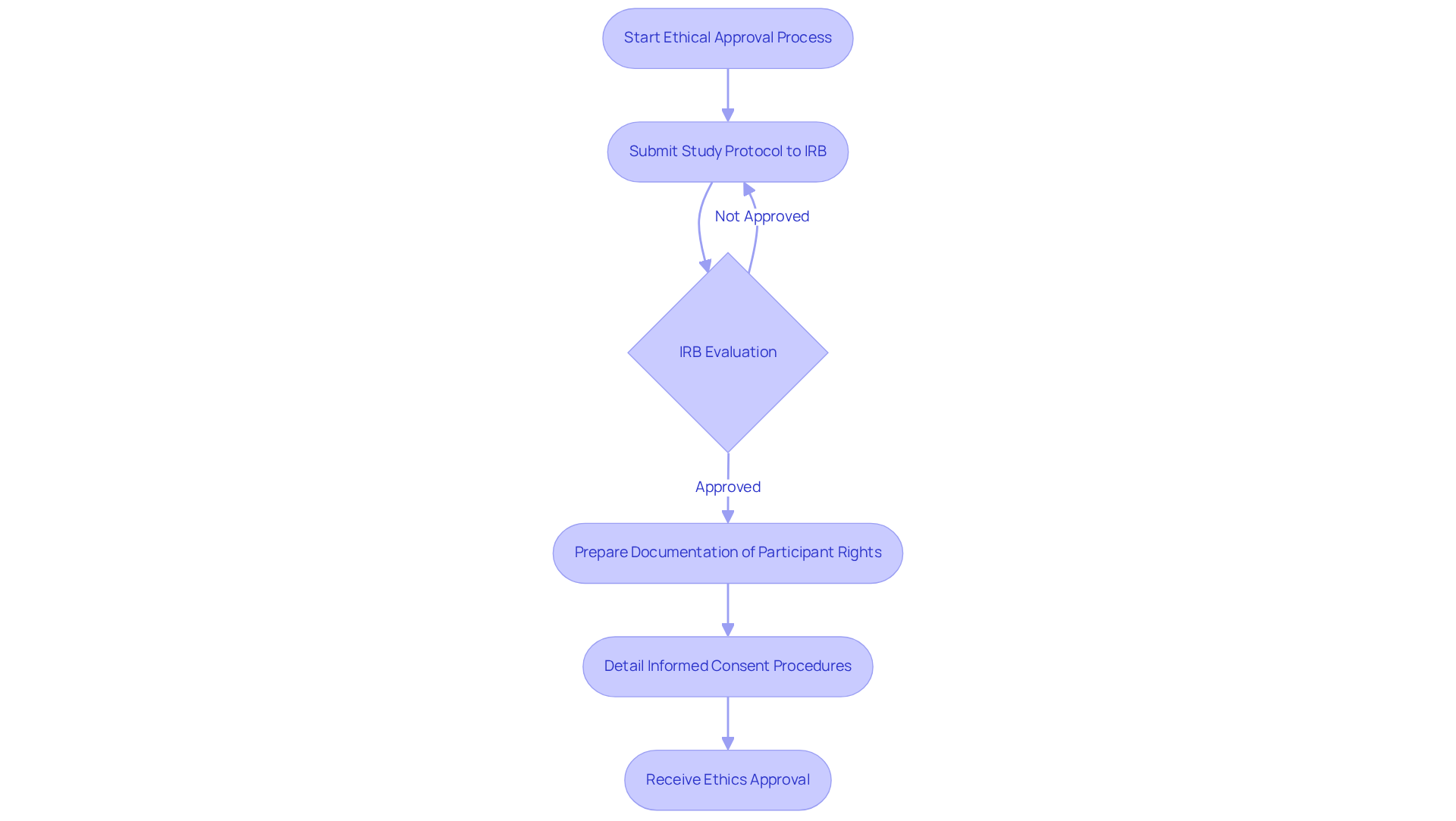
Obtaining ethical approvals is a crucial step in the medical study process, requiring the submission of study protocols to Institutional Review Boards (IRBs) or Ethics Committees for thorough evaluation. Clinical Research Directors must design studies that prioritize participant rights and welfare, ensuring that informed consent processes are both transparent and comprehensive.
Statistics reveal that the overall median time to activation for clinical trials is 234 days, with ethics approval taking a median of 48 days. This timeline underscores the importance of effective ethical supervision, which not only protects participants but also enhances the credibility and integrity of the study.
Effective IRB submissions typically include:
As one ethics committee member noted, "The aim is that participants engage in the study willingly, with a comprehension of what their involvement entails."
By adhering to structured steps to secure ethical approvals, including pre-submission meetings and the use of standardized forms, Clinical Research Directors can significantly increase the likelihood of timely approvals and successful trial outcomes.
Implementing robust quality control measures is essential for ensuring the reliability of IVD. This includes:
By fostering a culture of quality, Clinical Research Directors can significantly reduce errors, enhance data integrity, and ultimately improve patient outcomes. GCP adherence not only guarantees conformity with governing standards but also positively impacts study outcomes, as investigations conducted under these guidelines tend to produce more dependable and reproducible results.
Best practices for quality control in IVD studies in 2025 include:
Insights from industry leaders highlight that a commitment to quality control measures is paramount in navigating the complexities of IVD, ultimately leading to better diagnostic accuracy and patient care.

Encouraging cooperation among stakeholders—including researchers, sponsors, oversight organizations, and ethics committees—plays a crucial role in enhancing the effectiveness of IVD. Establishing clear communication channels and shared objectives enables Clinical Research Directors to streamline processes, minimize redundancies, and expedite decision-making. This collaborative approach not only accelerates study completion but also facilitates quicker product availability, ultimately benefiting patient access to innovative diagnostics.
Industry leaders emphasize that effective communication is essential; organizations prioritizing collaboration are five times more likely to be regarded as high-performing. Furthermore, successful communication strategies, such as regular updates and inclusive discussions, can significantly enhance stakeholder engagement and satisfaction. By leveraging these joint efforts, the impact on trial efficiency in IVD becomes evident, resulting in more prompt and effective progress in development.

The Clinical Laboratory Improvement Amendments (CLIA) set forth rigorous standards for laboratory testing, ensuring accuracy and reliability in results. For laboratories engaged in IVD studies, compliance with CLIA is not merely a requirement; it is a fundamental aspect of quality assurance. Adherence to these standards significantly enhances the trustworthiness of laboratory outcomes, which is crucial for study directors aiming to uphold the integrity of their investigations and avert potential regulatory fines.
Recent statistics reveal that since the implementation of CLIA '88, the percentage of laboratories passing proficiency testing (PT) has consistently increased, reflecting advancements in testing accuracy. For instance, data from 1994 to 2006 indicate a notable decline in failure rates for various analytes, highlighting the positive influence of CLIA compliance on laboratory performance.
As we approach 2025, laboratories must adapt to updated CLIA requirements, which entail enhanced qualifications for lab directors and stricter oversight of testing personnel. These modifications necessitate that clinical study directors ensure their laboratories not only meet but exceed these standards to maintain operational excellence.
Insights from laboratory experts underscore the significance of continuous quality improvement practices, such as regular competency evaluations and active participation in PT programs, to sustain high testing accuracy under CLIA. By cultivating a culture of compliance and quality, IVD laboratories can markedly enhance their testing outcomes, ultimately benefiting patient care and propelling IVD medical research forward.

The 510(k) premarket notification process stands as a crucial pathway for manufacturers to demonstrate that their IVD devices are substantially equivalent to existing legally marketed devices. For Clinical Research Directors, understanding the intricacies of 510(k) submissions is essential, requiring a thorough compilation of data on device performance, safety, and intended use. Recent trends reveal that nearly 32% of submissions did not pass the initial acceptance for review check, highlighting the necessity for meticulous preparation to circumvent delays and additional costs.
Navigating the 510(k) process successfully is vital for timely market entry, especially considering that the average time from application submission to FDA decision has remained stable at approximately 147 days. This timeline may extend if further information is requested, which is common; indeed, as of September 2022, 67% of submissions led to requests for additional information.
Case studies illustrate that 98.4% of devices cleared through the 510(k) process remained free of recall one year post-clearance, suggesting a generally reliable performance of devices that effectively navigate this pathway. Furthermore, understanding the prerequisites for demonstrating substantial equivalence is crucial, as it directly impacts the likelihood of obtaining approval and achieving market success.
As the landscape of IVD submissions evolves, staying informed about these dynamics will empower Clinical Research Directors to facilitate smoother compliance processes and enhance the chances of successful market entry for innovative IVD solutions.

Ensuring data integrity in research trials is paramount, hinging on the implementation of robust data management practices. This involves establishing secure data collection methods, essential for protecting sensitive information and maintaining participant trust. Regular data audits are crucial for identifying discrepancies and ensuring compliance with regulatory standards. Comprehensive training for staff on data handling procedures fosters a culture of accountability and precision.
Moreover, the extensive trial management services provided by bioaccess—encompassing feasibility studies, site selection, compliance assessments, trial setup, import permits, project oversight, and reporting—play a vital role in improving data integrity. Clinical Research Directors must prioritize these elements to uphold the credibility of their research findings.
Statistics reveal that nearly 49% of analyzed clinical papers exhibit data integrity concerns, underscoring the necessity for stringent data governance. Insights from industry leaders emphasize that effective data management not only enhances the reliability of trial outcomes but also accelerates the path to regulatory approval, ultimately contributing to the successful commercialization of innovative medical products.

The evolving landscape of in vitro diagnostics (IVD) presents a critical opportunity for Clinical Research Directors to enhance their understanding of compliance and regulatory frameworks. By grasping the complexities of IVD regulations, including the latest guidelines and classification systems, directors can ensure their research not only meets necessary standards but also fosters innovation in diagnostic solutions.
Key insights from the article underscore the importance of robust compliance strategies. Adhering to the In Vitro Diagnostic Regulation (IVDR), understanding the implications of IVD classifications, and navigating the Investigational Device Exemption (IDE) process are essential. Additionally, emphasizing:
significantly contributes to the success and reliability of clinical trials.
As the IVD market continues to grow, it is essential for Clinical Research Directors to stay informed and proactive. Engaging with platforms like bioaccess® can facilitate a smoother navigation of these complexities, ultimately accelerating clinical research and enhancing patient safety. By prioritizing these insights, directors can streamline their processes and drive the development of innovative diagnostics that meet the needs of a rapidly changing healthcare environment.
What is bioaccess® and how does it support Medtech startups?
bioaccess® empowers Medtech startups by leveraging its expertise in early-stage trials, particularly in Colombia, which is known for first-in-human assessments. It helps startups navigate clinical trials efficiently, offering significant cost reductions exceeding 30% compared to North America and Western Europe, and a regulatory review process that takes just 90-120 days.
Why is Colombia considered a favorable location for clinical trials?
Colombia is considered a favorable location for clinical trials due to its high-quality healthcare system, a diverse patient population of over 50 million (with 95% covered by universal healthcare), and hospitals that conduct research only after achieving stringent ICH/GCP certification. This ensures rigorous quality standards in clinical research.
What are the advantages of conducting clinical trials in Colombia?
Advantages of conducting clinical trials in Colombia include significant cost reductions, a fast regulatory review process, access to a large patient population, and appealing R&D tax incentives such as substantial tax deductions and grants for innovative projects.
What are the key compliance requirements for IVD regulations?
Key compliance requirements for IVD regulations include producing strong medical evidence to support performance claims and conducting well-structured studies or reassessing existing data. Compliance with these regulations is critical for ensuring that diagnostic devices meet safety and efficacy standards before market entry.
What is the IVDR and how does it affect IVD devices?
The IVDR, or In Vitro Diagnostic Regulation, came into effect on May 26, 2022, and introduces a risk-based classification system for IVD devices into four classes (A, B, C, and D). This affects the conformity assessment process and the level of scrutiny required for CE marking, making compliance more stringent.
How does the classification of IVD devices impact clinical research?
The classification of IVD devices impacts clinical research by determining the type of data needed for regulatory approval, influencing study design, and affecting the overall timeline for product commercialization. Higher-risk classes require more rigorous compliance measures.
What challenges do companies face under the new IVDR regulations?
Companies face challenges under the new IVDR regulations due to the heightened scrutiny and higher standards of medical evidence required compared to the previous IVDD. Many companies lack the necessary evidence for compliance, complicating the approval process.
How can bioaccess® assist in navigating IVD compliance?
bioaccess® can assist in navigating IVD compliance by providing trial management services that include feasibility studies, site selection, compliance reviews, trial setup, import permits, project management, and detailed reporting on study status and adverse events.
What is the significance of staying informed about IVD regulations for Clinical Research Directors?
Staying informed about IVD regulations is significant for Clinical Research Directors as it helps them mitigate risks, enhance patient safety, and ensure the efficacy of IVDs. Understanding the latest updates and trends can lead to successful market entry for their products.