


The article emphasizes the identification of essential tools for multi-language dossier publishing in Brazil, a critical factor in enhancing the efficiency and compliance of clinical trials. It presents various platforms and services, including:
These tools collectively streamline processes, improve compliance, and ultimately accelerate approval timelines for clinical submissions within the Brazilian market.
The burgeoning clinical trials market in Brazil, anticipated to reach USD 2.44 billion by 2034, offers a distinctive opportunity for organizations aiming to navigate the intricacies of multi-language dossier publishing. As regulatory landscapes shift and the demand for innovative medical solutions escalates, utilizing the appropriate tools becomes crucial for achieving success.
However, with various challenges—including compliance hurdles and language barriers—how can companies guarantee efficient and effective dossier management? This article delves into seven essential tools that not only streamline the publishing process but also bolster the chances of successful regulatory submissions in Brazil.
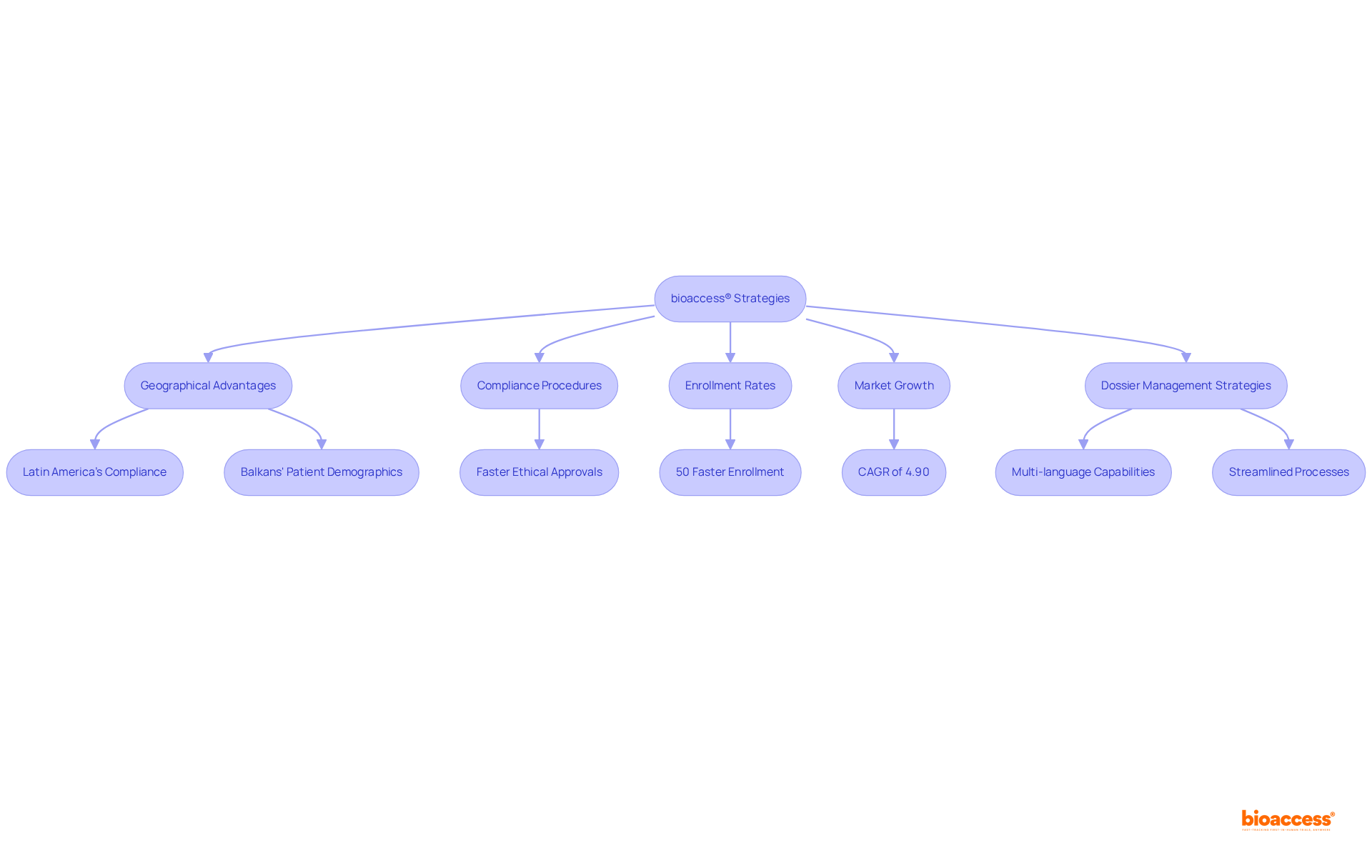
bioaccess® excels in the clinical research landscape, delivering exceptional agility in dossier publishing. Focusing on Medtech, Biopharma, and Radiopharma, bioaccess® leverages Latin America's swift compliance procedures, the Balkans' diverse patient demographics, and Australia's effective pathways to secure ethical approvals within 4-6 weeks. This remarkable speed translates to enrollment rates that are 50% faster than those in traditional markets, positioning bioaccess® as a vital ally for innovators seeking to accelerate their clinical trials in Brazil and beyond.
The Brazilian clinical trials market, valued at USD 1.51 billion in 2024, is projected to grow significantly at a CAGR of 4.90%, reaching USD 2.44 billion by 2034. This growth is driven by increasing investments in biotechnology and enhanced regulatory support. Consequently, the effectiveness of document publishing becomes crucial for success. Recent advancements in this area have focused on streamlining processes, including the integration of digital tools and improved communication strategies to meet the demands of a rapidly evolving healthcare landscape.
Effective strategies for multi-language dossier publishing Brazil tools require a comprehensive understanding of local regulations and the incorporation of multilingual capabilities to serve various stakeholders. Medtech leaders emphasize that efficient dossier management not only accelerates the approval process but also enhances the overall success rates of clinical trials. By adopting these strategies, bioaccess® ensures that its clients can navigate the complexities of the Brazilian market with confidence, ultimately leading to faster commercialization of innovative medical solutions.
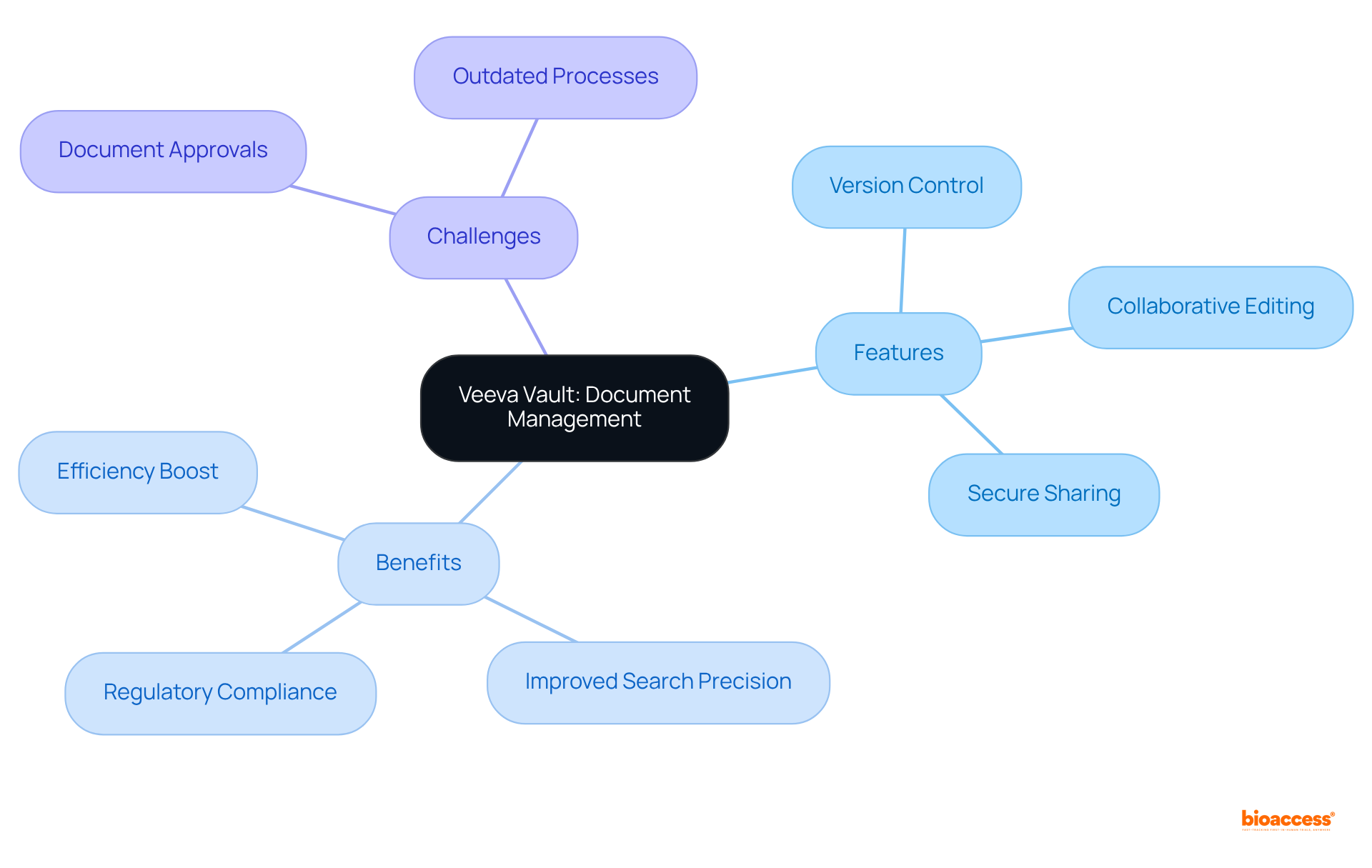
Veeva Vault presents a comprehensive platform for managing compliance filings, ensuring that all documents are systematically organized, readily accessible, and compliant with local regulations. With features such as version control and collaborative editing, Veeva Vault directly addresses the challenges that 66% of companies encounter in document approvals and reviews, often arising from outdated processes. By leveraging Veeva Vault, organizations can significantly boost their efficiency in using multi-language dossier publishing Brazil tools for preparing filings for regulatory bodies.
The platform's advanced capabilities, including secure sharing and an anticipated 90% improvement in document search precision through machine learning by 2024, further streamline workflows and enhance adherence to evolving compliance standards. As the landscape of regulatory compliance continues to evolve, Veeva Vault stands out as an indispensable resource for enterprises aiming to navigate these challenges effectively.
MasterControl emerges as a premier quality management system, empowering organizations to maintain compliance with regulatory standards throughout the multi-language dossier publishing Brazil tools process. Its automated solutions not only streamline quality processes but also manage documentation efficiently and facilitate audits—crucial elements in meeting the stringent requirements set forth by Brazilian authorities.
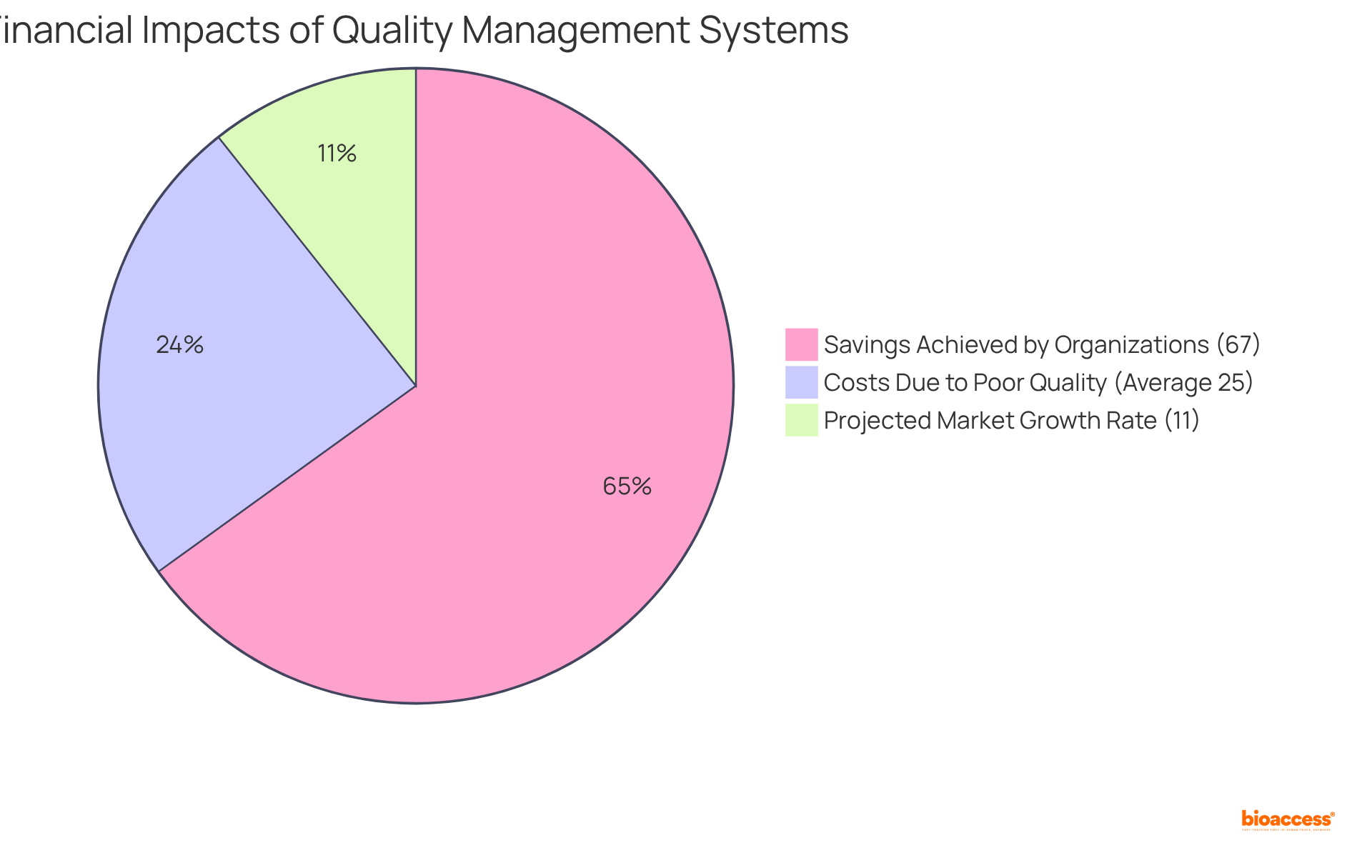
By integrating MasterControl into their operational workflows, companies can significantly reduce the risk of non-compliance, thereby enhancing the overall quality of their submissions. Indeed, organizations that adopt robust quality management systems frequently report considerable savings, with 67% achieving at least $25,000 in savings within one year. This is particularly vital in a landscape where the cost of poor quality can represent 15 to 35 percent of total business costs, highlighting the necessity of effective quality management in attaining operational excellence.
Furthermore, the global quality management software market is projected to expand at a CAGR of 11.0%, indicating the growing acknowledgment of quality management's influence on operational efficiency and customer satisfaction.
As compliance expert William Foster aptly stated, "Quality is never an accident; it is always the result of high intention, sincere effort, intelligent direction, and skillful execution." This underscores the critical role of quality management systems like MasterControl for companies navigating the complexities of multi-language dossier publishing Brazil tools.
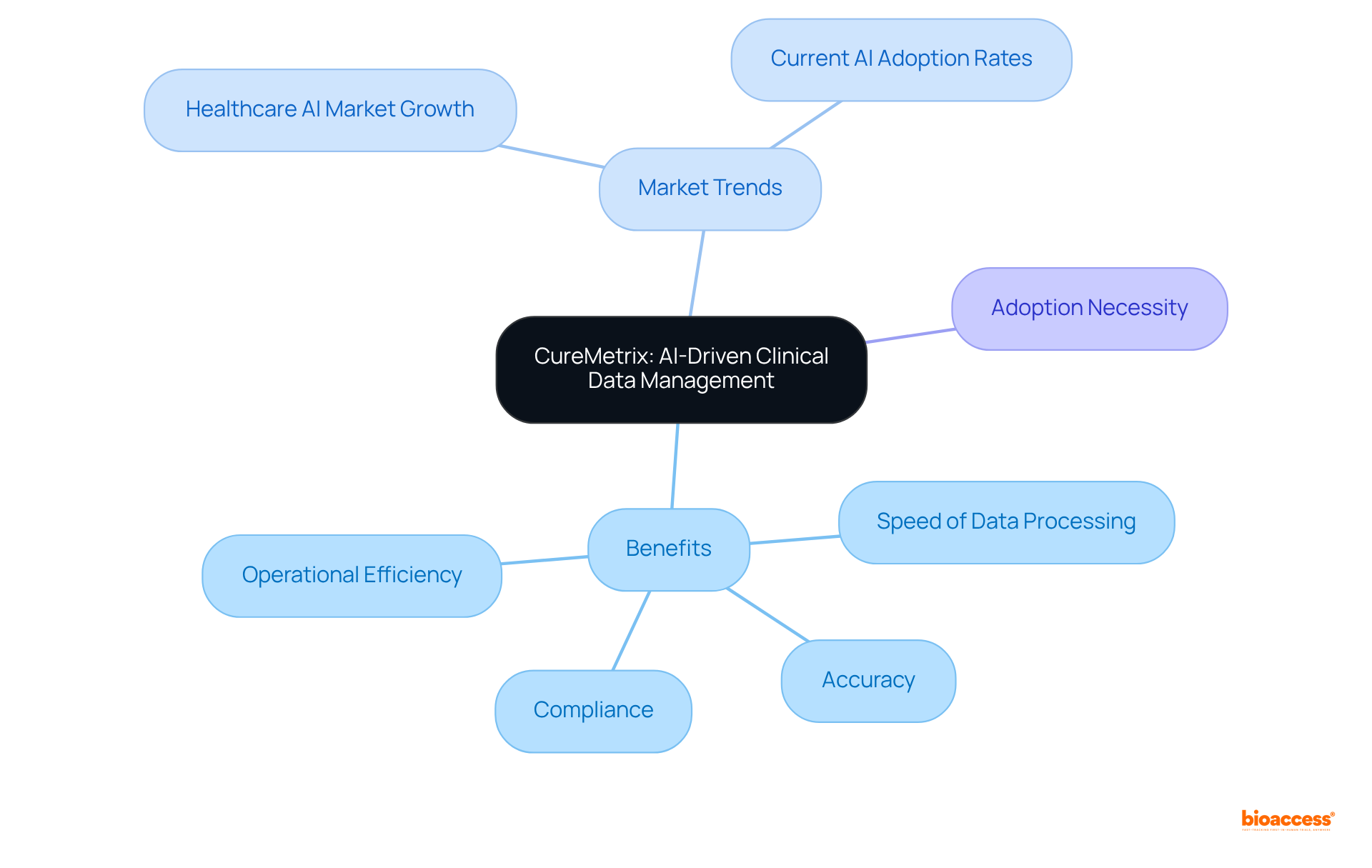
CureMetrix leverages artificial intelligence to revolutionize clinical data management, significantly enhancing the speed and accuracy of data processing. This innovative technology is particularly beneficial for entities involved in multi-language dossier publishing Brazil tools, as it simplifies tasks related to data entry and validation. By adopting CureMetrix, companies can ensure their clinical data meets compliance standards while being readily accessible for regulatory submissions using multi-language dossier publishing Brazil tools. The integration of AI not only accelerates data processing times but also enhances overall efficiency, enabling companies to focus on advancing their clinical trials and attaining timely market access.
Moreover, with the healthcare AI market expected to expand considerably, and 86% of healthcare entities currently employing AI extensively, the adoption of CureMetrix positions companies at the forefront of this transformative trend. As highlighted by industry leaders, AI's role in alleviating administrative burdens and boosting operational efficiency is vital for entities striving to succeed in the competitive realm of clinical research. The imperative is clear: to thrive in this evolving landscape, embracing AI-driven solutions like CureMetrix is not merely advantageous; it is essential.
Medidata Solutions presents a powerful cloud-based platform designed to enhance the efficiency of clinical trials and compliance processes. This suite of multi-language dossier publishing Brazil tools facilitates effective data gathering, management, and analysis, which are critical elements for organizations preparing documentation for submission to Brazilian oversight bodies. By leveraging Medidata's solutions, companies can markedly elevate their operational efficiency while ensuring compliance with local regulations. This approach not only accelerates the submission process but also resonates with contemporary trends in data management, underscoring the importance of real-time data access and collaborative efforts in compliance submissions.
The Regulatory Affairs Professionals Society (RAPS) serves as a vital resource for compliance specialists, offering extensive training opportunities essential for mastering the complexities of document publishing. Engaging with RAPS allows professionals to stay abreast of evolving regulations and best practices, which are necessary for utilizing multi-language dossier publishing Brazil tools in the dynamic governance landscape.
The training programs provided by RAPS have significantly enhanced compliance rates in presentations through the use of multi-language dossier publishing Brazil tools, equipping professionals with the critical skills needed to navigate compliance standards effectively. Notably, the global clinical trial service market reached $64 billion by 2020, highlighting the industry's scale and the paramount importance of compliance.
Furthermore, RAPS consistently updates its offerings to incorporate the latest training opportunities, ensuring that members are well-prepared to address the challenges of submissions. Success stories from professionals who have benefited from RAPS training underscore the positive impact of such resources on achieving compliance and advancing their careers.
As Allison Komiyama, a Regulatory Consultant, articulates, "For me, there's no feeling quite like helping get a medical device to market, and in doing so, helping to make people's lives better." This statement underscores the essential role RAPS plays in supporting professionals throughout their regulatory journeys.
DocuSign revolutionizes the document approval process by providing electronic signature solutions that significantly enhance efficiency for organizations in Brazil. By adopting DocuSign, companies can drastically reduce the time spent on manual signatures, which traditionally hinders the approval of essential dossiers.
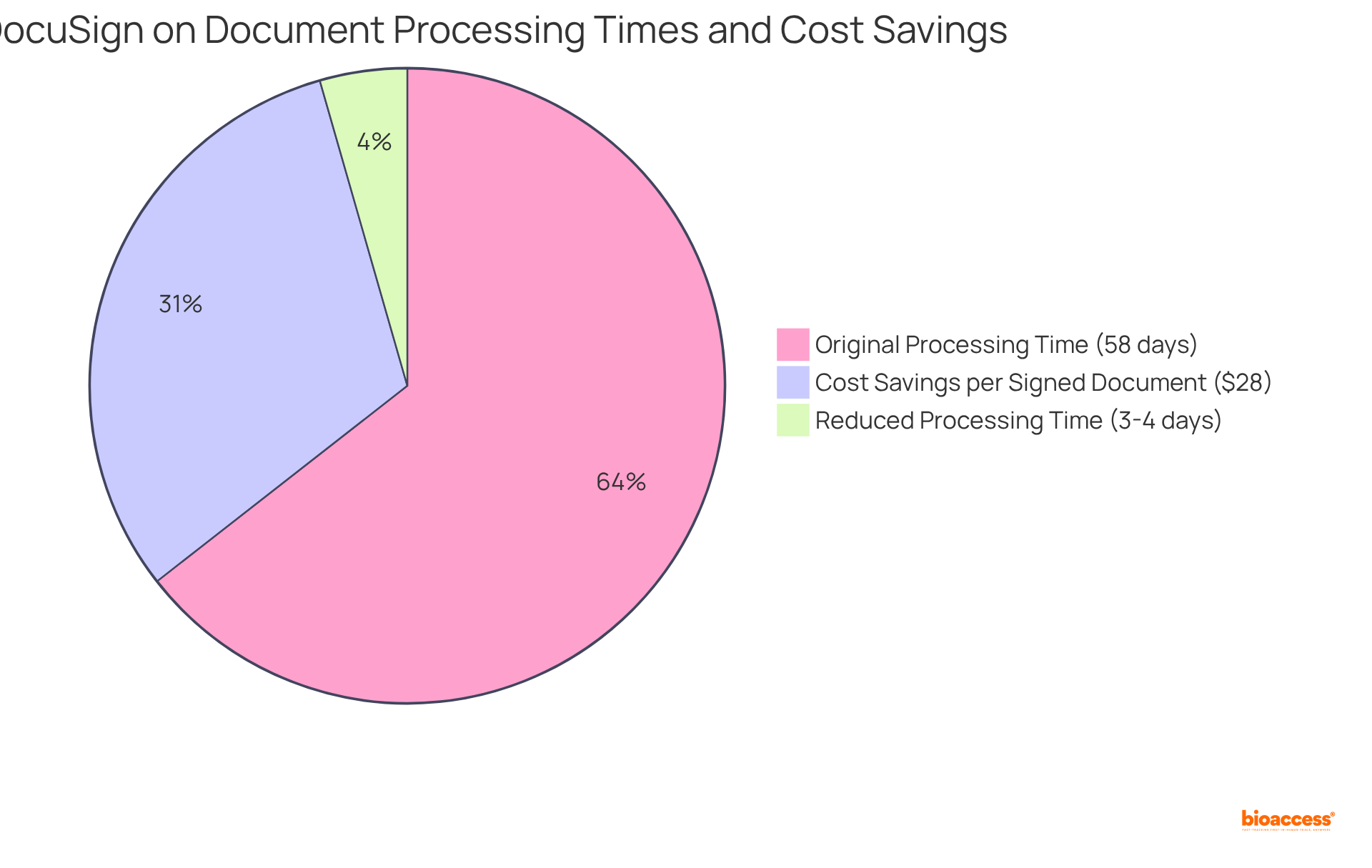
Studies indicate that implementing electronic signatures can reduce document processing times from up to 58 days to just 3 to 4 days, allowing clinical trials to progress more swiftly. This streamlined method not only accelerates the approval timeline but also ensures compliance with legal requirements, a crucial element in the fast-paced clinical research environment.
As industry leaders emphasize, the ability to expedite approvals is essential for maintaining competitiveness and meeting the demands of a rapidly evolving market. Furthermore, companies can save an average of $28 per signed document, underscoring the financial benefits of adopting electronic signatures.
The successful incorporation of DocuSign across various entities demonstrates its effectiveness in transforming document workflows, ultimately resulting in quicker compliance submissions and enhanced operational efficiency. Moreover, the ecological advantages of utilizing e-signatures, such as the potential to conserve 2.5 billion trees over two decades, resonate with the increasing focus on sustainability among enterprises.
Overall, DocuSign not only enhances efficiency but also aligns with the values of corporate responsibility.
PharmaLex provides specialized compliance services along with multi-language dossier publishing Brazil tools specifically tailored for biopharma companies operating in Brazil. Their extensive understanding of the intricate compliance landscape empowers organizations to adeptly navigate submission processes, addressing the prevalent adherence challenges that many biopharma firms face, such as adapting to evolving regulations and ensuring timely submissions. By collaborating with PharmaLex, companies can markedly enhance their compliance strategies, thereby increasing their likelihood of securing successful approvals.
Additionally, bioaccess® bolsters these efforts with its expertise in multi-language dossier publishing Brazil tools and compliance writing, providing a comprehensive support system for biopharma entities. As Paul Koziarz, President and General Manager of Regulatory Compliance at CSI, asserts, "Without compliance, many organizations wouldn’t have security controls in place, and there would be no consistency of standards among the protocols being used."
This partnership is crucial as the biopharma sector continues to evolve, necessitating robust strategies to effectively manage the complexities of compliance demands. To strengthen compliance, biopharma firms should consider:
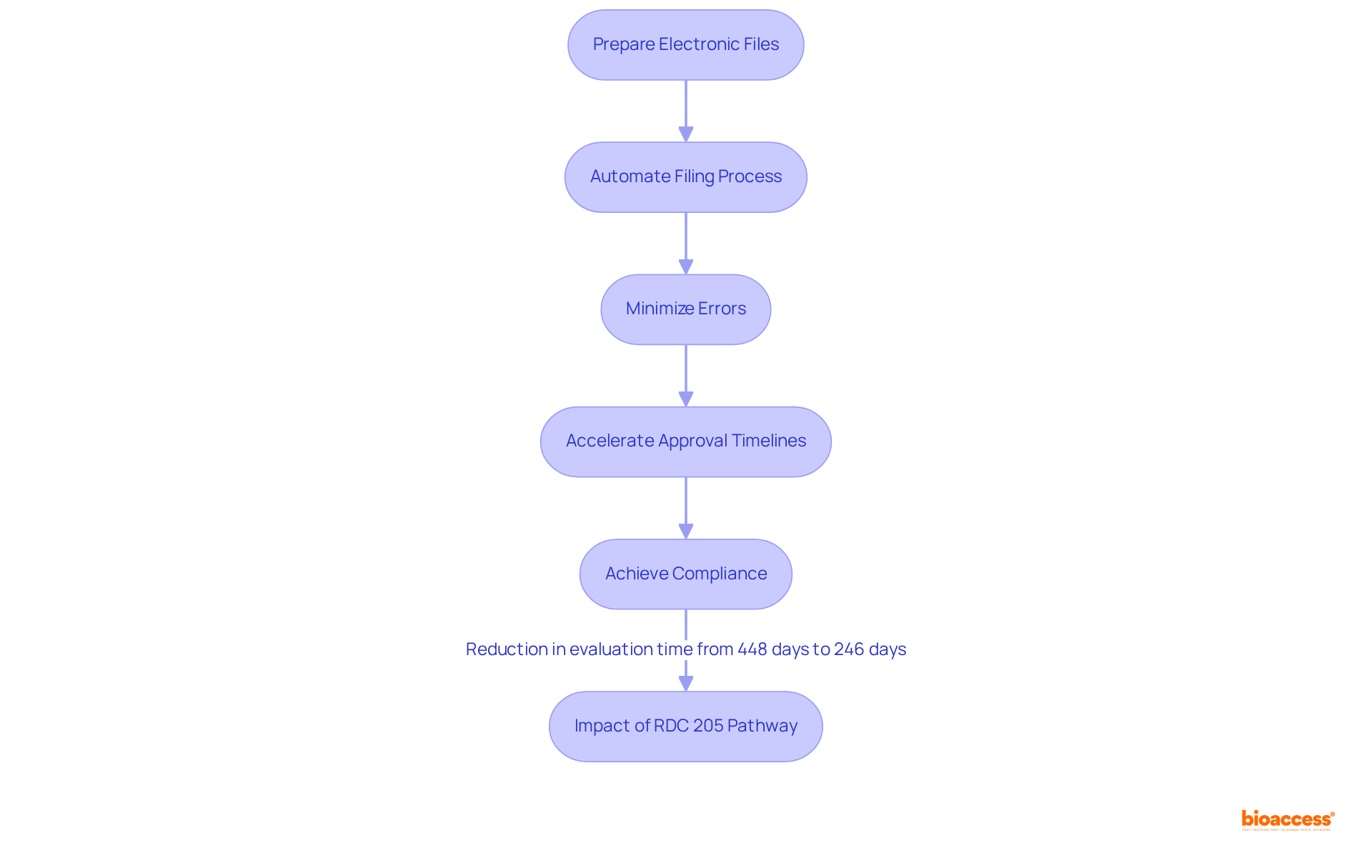
The eCTD application software significantly streamlines the preparation and delivery of electronic files to regulatory bodies in Brazil. By automating various elements of the filing process, this software minimizes the risk of errors and accelerates approval timelines. Notably, since the introduction of the RDC 205 pathway, the average evaluation time for rare disease therapies by ANVISA has been reduced to 246 days—nearly half the duration of the previous process, which could take up to 448 days.
Organizations looking to enhance their capabilities in multi-language dossier publishing Brazil tools should strongly consider adopting eCTD filing software to ensure compliance and improve operational efficiency. As industry leaders emphasize, the transition to electronic filings not only facilitates quicker access to therapies but also aligns with the growing demand for flexibility in oversight procedures.
Marcela Vega asserts, "The Ministry of Health and payers in Brazil will have the opportunity to access new therapies for rare diseases earlier and with a locally controlled price." This digital transformation is essential for navigating the complexities of compliance filings and achieving timely market access.
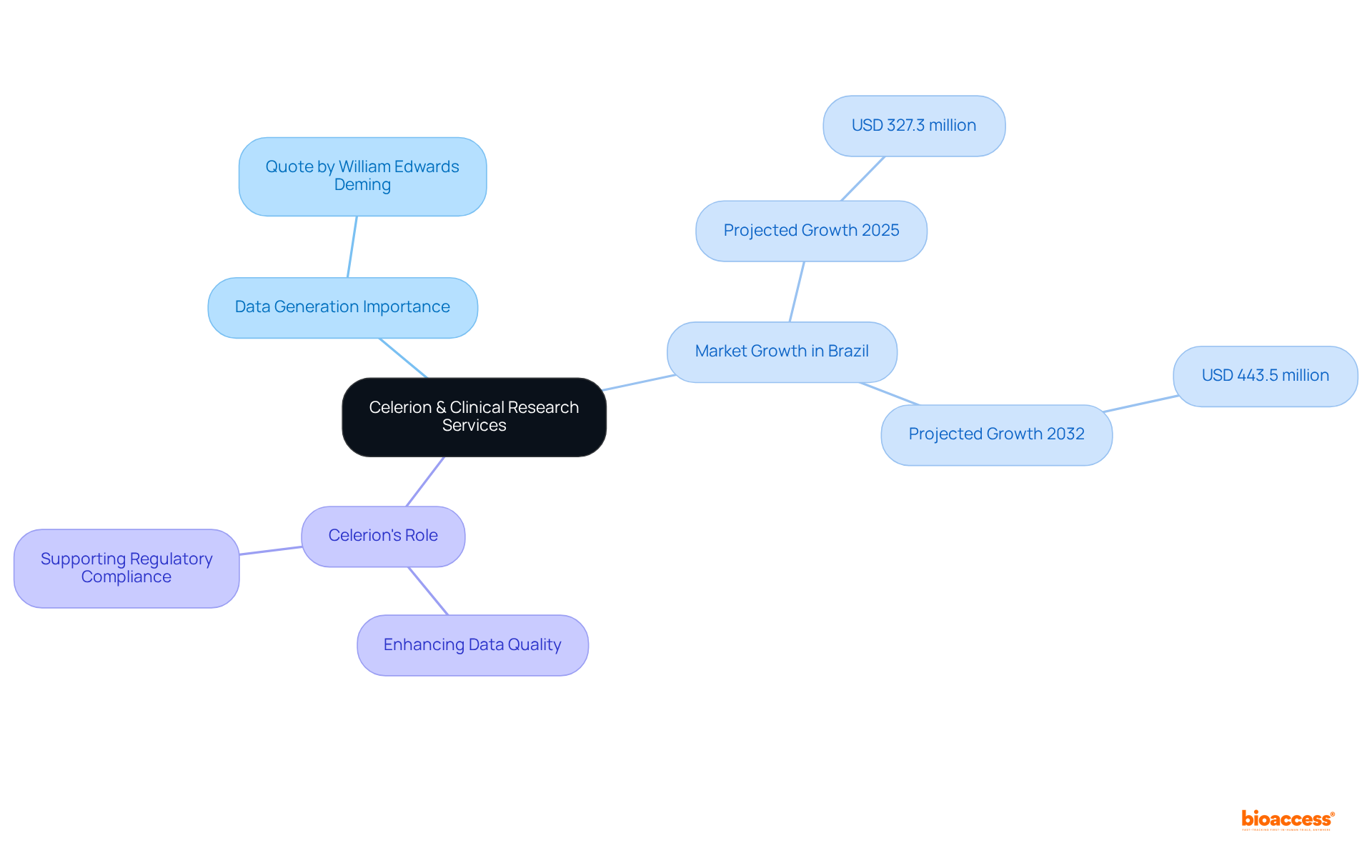
Celerion stands as a leader in clinical research services, adept at facilitating data generation for publication. Their expertise in early-phase clinical trials ensures that organizations secure high-quality data, a critical component for compliance processes.
In Brazil, the clinical trials market is poised for significant growth, projected to rise from USD 327.3 million in 2025 to USD 443.5 million by 2032. This trend underscores the escalating demand for effective data generation strategies.
By partnering with Celerion, companies can refine their data generation processes, thereby enhancing the quality of submissions to Brazilian authorities. As William Edwards Deming aptly stated, 'In God we trust; all others must bring data,' highlighting the paramount importance of data quality in regulatory submissions.
Celerion's unwavering commitment to excellence in data generation establishes them as an indispensable ally for organizations navigating the complexities of multi-language dossier publishing Brazil tools.
The landscape of multi-language dossier publishing in Brazil is increasingly complex, necessitating the adoption of specialized tools and strategies to ensure compliance and efficiency. Organizations aiming to thrive in this environment must leverage innovative solutions that streamline processes, enhance data management, and facilitate regulatory submissions. The essential tools discussed, ranging from bioaccess® to Celerion, underscore the importance of integrating advanced technology and expert knowledge to navigate the evolving regulatory framework effectively.
Key insights from the article highlight the significance of platforms like Veeva Vault and MasterControl in managing documentation and maintaining compliance standards. AI-driven solutions such as CureMetrix provide a competitive edge in clinical data management. Additionally, the role of organizations like RAPS and PharmaLex in offering training and compliance services further reinforces the need for continuous professional development in regulatory affairs. The incorporation of electronic signature solutions through DocuSign exemplifies how technology can transform traditional processes, resulting in faster approvals and improved operational efficiency.
Ultimately, the journey of multi-language dossier publishing in Brazil is not merely about meeting regulatory requirements; it is about fostering innovation and enhancing patient access to new therapies. As the clinical trials market continues to grow, embracing these essential tools and best practices will be crucial for organizations striving to succeed in a dynamic and competitive landscape. Taking proactive steps toward integrating these solutions can lead to significant advancements in compliance, efficiency, and ultimately, the successful commercialization of medical innovations.
What is bioaccess® and what does it offer in the clinical research landscape?
bioaccess® excels in dossier publishing within the clinical research landscape, particularly for Medtech, Biopharma, and Radiopharma sectors. It leverages rapid compliance procedures in Latin America, diverse patient demographics in the Balkans, and effective pathways in Australia to secure ethical approvals in 4-6 weeks, resulting in enrollment rates that are 50% faster than traditional markets.
What is the projected growth of the Brazilian clinical trials market?
The Brazilian clinical trials market is valued at USD 1.51 billion in 2024 and is projected to grow at a CAGR of 4.90%, reaching USD 2.44 billion by 2034, driven by increased investments in biotechnology and enhanced regulatory support.
How does document publishing affect clinical trial success?
Effective document publishing is crucial for success in clinical trials. Recent advancements focus on streamlining processes through digital tools and improved communication strategies, which help meet the demands of a rapidly evolving healthcare landscape.
What strategies does bioaccess® employ for multi-language dossier publishing in Brazil?
bioaccess® employs strategies that include a comprehensive understanding of local regulations and the integration of multilingual capabilities to serve various stakeholders, enhancing the approval process and overall success rates of clinical trials.
What is Veeva Vault and how does it support regulatory submissions?
Veeva Vault is a comprehensive platform for managing compliance filings, ensuring systematic organization, accessibility, and compliance of documents with local regulations. It features version control and collaborative editing to address challenges in document approvals and reviews.
How can Veeva Vault improve document management efficiency?
By leveraging Veeva Vault, organizations can significantly enhance their efficiency in using multi-language dossier publishing tools, with anticipated improvements in document search precision through machine learning by 2024.
What role does MasterControl play in quality management for dossier publishing?
MasterControl is a premier quality management system that helps organizations maintain compliance with regulatory standards throughout the dossier publishing process. It automates quality processes, manages documentation efficiently, and facilitates audits.
What are the financial benefits of adopting quality management systems like MasterControl?
Organizations that implement robust quality management systems often report significant savings, with 67% achieving at least $25,000 in savings within one year, highlighting the importance of effective quality management in reducing costs associated with poor quality.
What is the projected growth of the global quality management software market?
The global quality management software market is projected to expand at a CAGR of 11.0%, reflecting the increasing recognition of quality management's impact on operational efficiency and customer satisfaction.