


The article titled "7 Key CDMS Medical Abbreviation Insights for Clinical Research" underscores the critical role of Clinical Data Management Systems (CDMS) in enhancing the efficiency and integrity of clinical research.
It articulates how CDMS solutions effectively streamline data collection, validation, and analysis. This not only ensures regulatory compliance but also significantly improves the success rates of research initiatives.
Case studies illustrate these benefits, showcasing:
Thereby reinforcing the importance of CDMS in the Medtech landscape.
The landscape of clinical research is evolving rapidly, propelled by the imperative for efficiency and accuracy in data management. As the demand for streamlined processes intensifies, the importance of Clinical Data Management Systems (CDMS) becomes increasingly clear, providing solutions that bolster the integrity and reliability of research data. Yet, amid the complexities of modern studies and heightened regulatory scrutiny, how can organizations ensure they are effectively leveraging CDMS to navigate these challenges? This article explores seven key insights regarding CDMS and its pivotal role in transforming clinical research practices.
bioaccess® leverages its extensive expertise across Latin America, the Balkans, and Australia to deliver exceptional management services for health information. With ethical approvals secured in an impressive timeframe of 4-6 weeks, and patient enrollment rates soaring by over 50% compared to traditional markets, bioaccess® markedly improves the efficiency of studies for Medtech, Biopharma, and Radiopharma innovators. This swift response is crucial in a healthcare environment where approximately 3,700 research studies receive approval annually in the EU, highlighting the growing demand for rapid and reliable information management.
Industry leaders emphasize that the adoption of advanced technologies and streamlined processes is vital for maintaining a competitive edge in research. As the complexity of research study endpoints has surged by 86% over the past decade, effective data management becomes increasingly essential to ensure study success. Notably, successful case studies, such as Avantec Vascular's first-in-human trial in Latin America for an innovative vascular device, demonstrate how bioaccess®'s tailored approach not only meets regulatory requirements but also fosters innovation, ultimately leading to enhanced health outcomes.
Furthermore, bioaccess® has established pre-qualified networks with over 50 locations activated in under 8 weeks, ensuring compliance with FDA/EMA/MDR regulations and facilitating expedited patient recruitment and site activation services for productive studies. Additionally, bioaccess® collaborates with organizations like the Caribbean Health Group to enhance its service offerings and position Barranquilla as a key hub for research studies in Latin America.
The CDMS medical abbreviation refers to a Clinical Data Management System, which is an essential software application that streamlines the management of information generated during research studies. The cdms medical abbreviation facilitates the collection, validation, and analysis of medical data, ensuring accuracy and compliance with regulatory standards. The CDMS market is poised for significant growth, reflecting the increasing reliance on CDMS solutions to navigate the complexities of modern research studies.
The importance of the cdms medical abbreviation in research studies is paramount. It plays a critical role in safeguarding data integrity, which is vital for the success of research initiatives. As regulatory scrutiny intensifies, pharmaceutical and biotechnology companies are increasingly turning to solutions referred to by the cdms medical abbreviation to guarantee data accuracy and compliance, thereby enabling timely submissions to regulatory authorities.
Effective medical studies that utilize the cdms medical abbreviation technology have demonstrated enhanced efficiency and data management capabilities. For instance, bioaccess® offers comprehensive clinical study management services that include:
This holistic approach accelerates patient enrollment—achieving recruitment that is 50% faster—and results in substantial cost savings of $25,000 per patient with FDA-ready data, eliminating rework and delays.
Recent advancements in the CDMS landscape further underscore its significance. The emergence of innovative platforms, such as Veeva SiteVault CTMS, illustrates the trend towards integrating features that enhance data flow between sites and sponsors, thereby improving overall study management. Experts note that the shift towards decentralized and hybrid evaluations heavily relies on real-time data collection and integration across various remote locations, making the cdms medical abbreviation a crucial component of contemporary studies.
In conclusion, the CDMS market is characterized by a dynamic competitive landscape, with established leaders and emerging innovators focusing on refining data management processes. As the demand for effective management of complex research data continues to rise, the CDMS medical abbreviation solutions, particularly those offered by bioaccess®, will play a pivotal role in shaping the future of medical studies.

A Case Report Form (CRF) serves as a pivotal document in the realm of clinical research, facilitating the collection of essential information from participants, including patient demographics, treatment protocols, and outcomes. The design of a CRF is paramount; it must align seamlessly with the study protocol to guarantee consistent and precise information collection, which is vital for the integrity of trial results.
Research demonstrates that a well-structured CRF can significantly reduce the time required to complete these forms, thereby enhancing overall efficiency in clinical research. For instance, the use of advanced information management tools has resulted in time savings of up to 99.4% compared to traditional methods.
Furthermore, the importance of CRF design cannot be overstated, as it directly influences the quality of analysis and the reliability of study outcomes. Experts in the field emphasize that employing optimal strategies in CRF development is essential for achieving robust and trustworthy results in research studies.
Electronic Data Capture (EDC) systems represent crucial digital platforms for the effective gathering and administration of research information. These systems significantly enhance the information gathering process, reducing errors linked to manual data entry. Studies demonstrate that the transition to EDC has led to a marked decrease in entry mistakes, thereby improving the overall accuracy of medical studies. Moreover, EDC systems provide real-time data access, enabling researchers to monitor and analyze information as it is collected, which is vital for timely decision-making.
The benefits of EDC systems extend beyond mere data collection; they play an essential role in enhancing the quality of medical studies. By facilitating comprehensive data management, EDC systems ensure that researchers can maintain high standards of data integrity and compliance with regulatory requirements. As highlighted by industry experts, the adoption of EDC systems is increasingly recognized as a critical factor for boosting efficiency in research, particularly in Phase III studies, which involve larger patient populations and significant data generation.
Moreover, bioaccess® accelerates studies by allowing treatment-naive cardiology or neurology groups to enroll 50% faster than their Western counterparts, achieving substantial cost savings of $25K per patient with FDA-ready data—no rework, no delays. This capability not only enhances the efficiency of medical studies but also addresses regulatory hurdles, facilitating quicker approvals for startups. Additionally, bioaccess® offers extensive management services for studies, including:
All of which further streamline the study process.
The growing emphasis on decentralized research underscores the importance of EDC systems in enhancing patient engagement and data quality. Overall, EDC systems signify a significant advancement in the digitization of research processes, ultimately yielding faster and more reliable outcomes. To harness these benefits, study directors should consider integrating EDC systems into their workflows to improve data management and ensure regulatory compliance, while also benefiting from the comprehensive management services provided by bioaccess®.
Standard Operating Procedures (SOPs) are essential, comprehensive documents that delineate the necessary procedures and practices in medical studies. They serve as a framework to ensure that all team members perform tasks consistently and adhere to regulatory standards, which is vital for maintaining the integrity of research. SOPs encompass various aspects of research studies, including participant recruitment, information management, and reporting, thereby significantly enhancing the reliability and quality of trial results.
The significance of SOPs in regulatory compliance cannot be overstated. They play a crucial role in upholding adherence to Good Clinical Practices (GCP) and other regulatory standards, which are fundamental for protecting participant rights and ensuring the integrity of information. Current compliance rates with SOPs in research are critical indicators of a site's operational efficiency, with studies showing that well-executed SOPs lead to improved compliance and superior quality data collection.
Effective SOPs not only direct daily operations but also act as educational resources for new staff, ensuring that everyone is aligned with established protocols. Regular training and updates are imperative to keep SOPs pertinent, as regulations and best practices evolve. For instance, a systematic approach to SOP creation, featuring clear objectives and comprehensive procedures, has been shown to foster a culture of adherence and elevate the overall quality of medical studies.
Successful trials frequently correlate with the effective implementation of SOPs. Research indicates that sites with robust SOP frameworks encounter fewer compliance challenges and cultivate better relationships with regulatory bodies. This is substantiated by case studies demonstrating that comprehensive SOPs yield improved outcomes during regulatory inspections, ultimately enhancing a site's reputation and competitiveness in future studies.
In conclusion, SOPs are the cornerstone of medical research, ensuring that studies are conducted ethically and effectively while safeguarding participant welfare and information integrity.
Good Clinical Practice (GCP) establishes the global ethical and scientific quality benchmarks that are essential for the design, execution, documentation, and reporting of research studies. These guidelines are pivotal in safeguarding the rights, safety, and welfare of participants while ensuring that the information produced is both reliable and accurate. Adhering to GCP is not merely a regulatory requirement; it is fundamental to maintaining the integrity of research and securing regulatory approvals. As research studies face increasing scrutiny, the importance of GCP compliance becomes even more pronounced, with organizations striving to tackle challenges such as evolving regulations and the need for robust data protection measures.
Comprehensive clinical study management services—including feasibility assessments, site selection, compliance evaluations, study setup, import permits, project management, and reporting—are instrumental in ensuring GCP adherence. Successful trials that conform to GCP standards not only enhance participant confidence but also contribute to the overall credibility of the scientific community. Furthermore, ongoing education and training in GCP are vital for all stakeholders involved, fortifying the commitment to ethical practices and the generation of high-quality data.

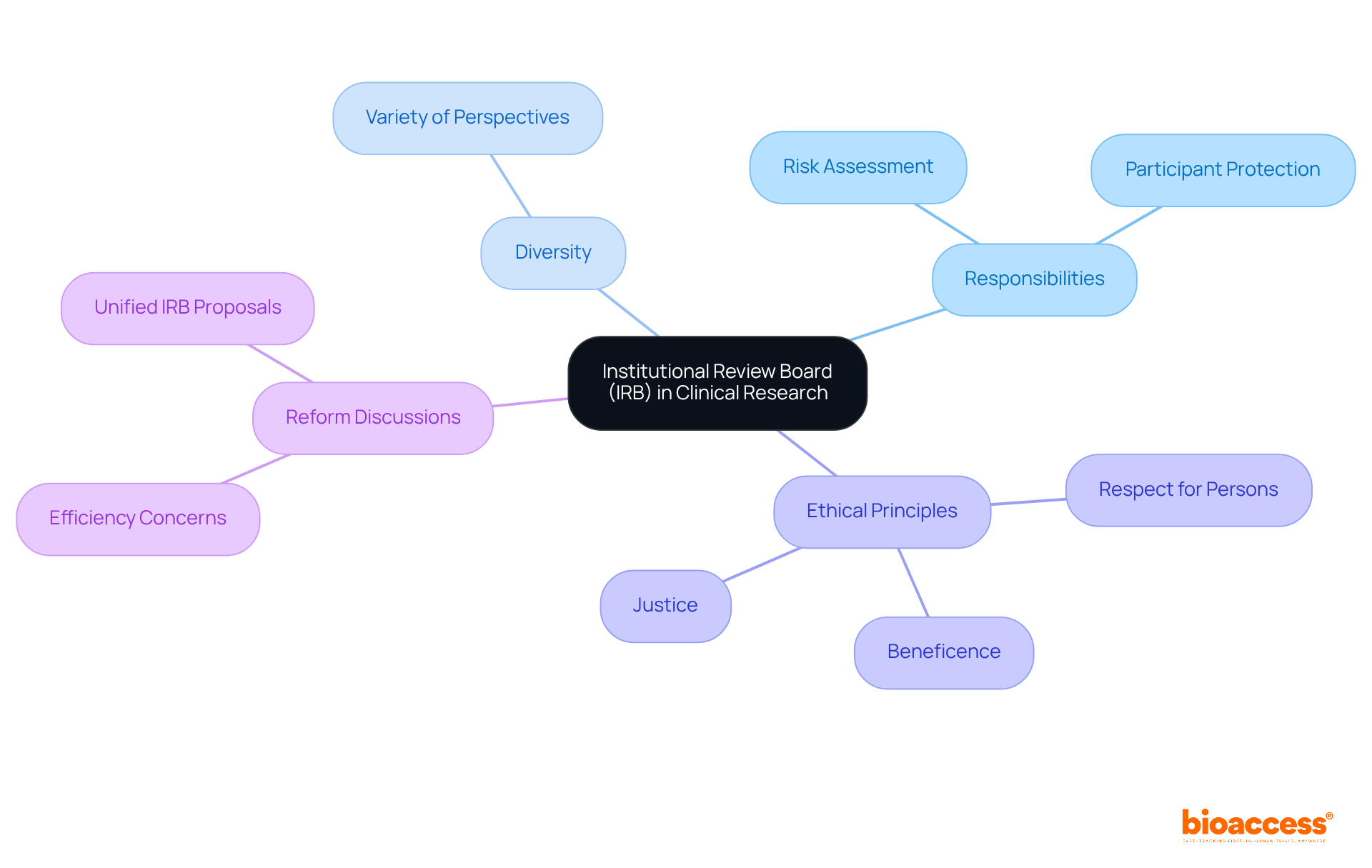
Institutional Review Boards (IRBs) serve as independent committees tasked with the ethical evaluation and endorsement of study protocols. Their primary responsibility is to assess the potential risks and benefits of research studies, ensuring the protection of participants' rights and welfare. This oversight is vital for maintaining public trust in clinical studies and ensuring adherence to ethical standards. As of May 2023, 77% of registered studies are interventional, with 40% focused on drugs or biologics, underscoring the crucial role IRBs play in supervising these investigations.
IRBs are required to include diverse members, ensuring a variety of perspectives in their evaluations. This diversity is essential, as it helps mitigate potential biases and enhances the ethical review process. The Belmont Report highlights the significance of respect for persons, beneficence, and justice in research—principles that IRBs are charged with upholding.
Recent discussions have centered on the necessity for reform in IRB processes, particularly concerning the efficiency of reviews. Critics argue that the current system can be overly bureaucratic, leading to delays in progress. Proposals for a unified IRB of record for multisite studies aim to streamline the review process while safeguarding participant protections. Despite these challenges, IRBs remain a cornerstone of ethical oversight in medical studies, ensuring that investigations are conducted transparently and responsibly.
Successful experiments often demonstrate the effectiveness of IRB oversight. For instance, the implementation of the Common Rule has standardized ethical review processes across federal agencies, thereby enhancing protections for human subjects. As the landscape of medical studies evolves, IRBs must adapt to new methodologies and challenges, ensuring that ethical standards are not only maintained but also improved in response to contemporary ethical issues.
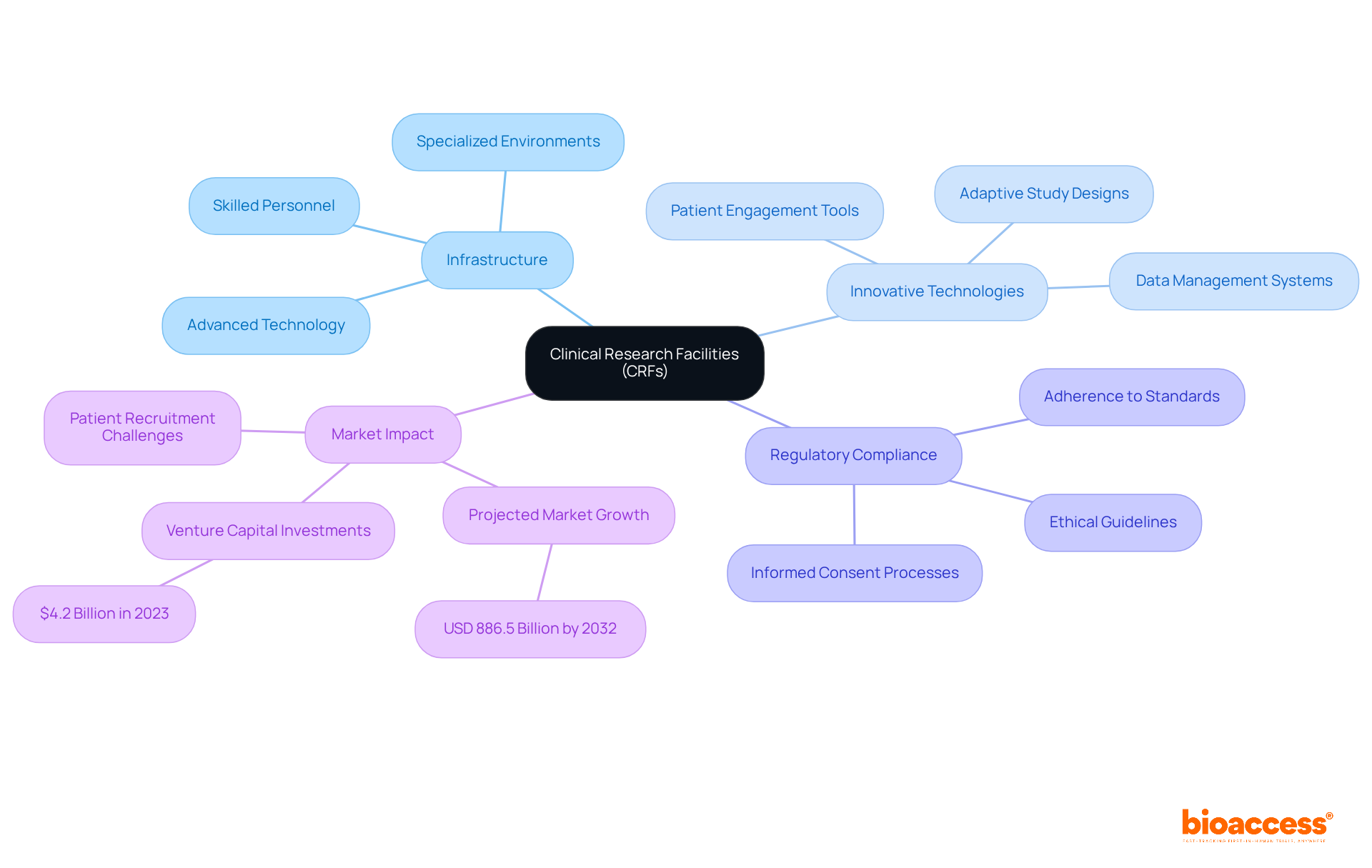
Clinical Research Facilities (CRFs) serve as specialized environments meticulously designed for conducting medical studies, equipped with the necessary infrastructure, advanced technology, and skilled personnel to facilitate comprehensive investigative activities. Their significance lies in their ability to ensure that studies are executed efficiently and adhere to stringent regulatory standards, which ultimately enhances the quality of research outcomes.
As the clinical research landscape evolves, CRFs are increasingly adopting innovative technologies and methodologies to streamline processes and improve participant engagement. Notably, the integration of adaptive study designs is gaining momentum, allowing for modifications based on interim findings without jeopardizing the integrity of the study.
Clinical study managers assert that 'the infrastructure provided by CRFs is essential for maintaining compliance and ensuring the integrity of data gathered during studies.' The successful execution of studies within specialized CRFs has illustrated the efficacy of this model, highlighting how dedicated facilities can expedite recruitment and bolster participant retention, thereby accelerating the journey to market for new therapies.
With the research studies market projected to reach approximately USD 886.5 billion by 2032, the role of CRFs will remain pivotal in shaping the future of medical research.
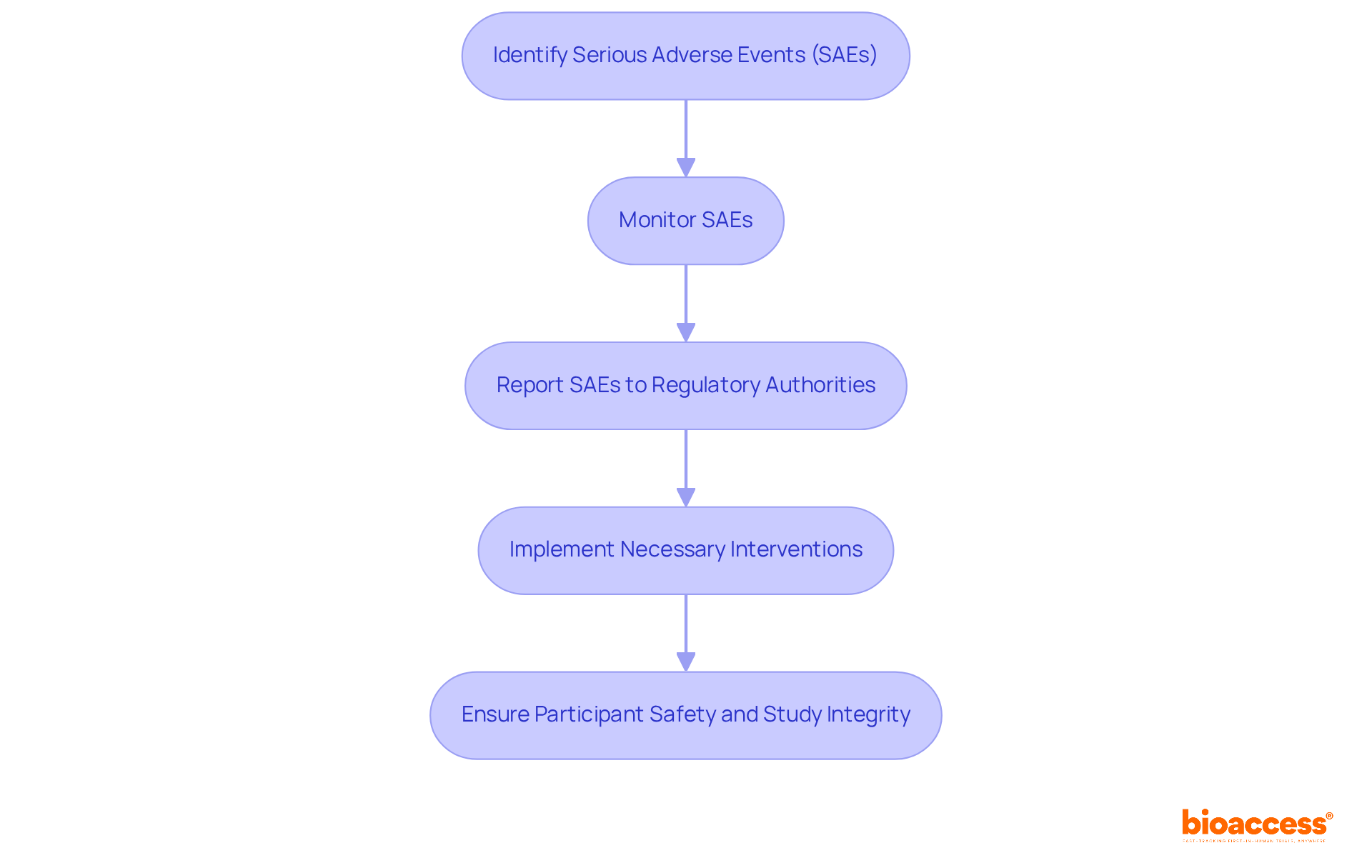
Monitoring Serious Adverse Events (SAEs) is a fundamental aspect of research management, as these occurrences can lead to severe outcomes, including hospitalization or death. Effective monitoring and timely reporting of SAEs are crucial for safeguarding participant safety and maintaining the integrity of the study. Regulatory authorities mandate that SAEs be reported promptly, allowing for necessary interventions to protect participants. Experts stress that comprehensive SAE monitoring not only improves participant safety but also aids in the overall success of medical studies.
Current reporting rates of SAEs in research studies suggest a need for enhancement, as numerous investigations still fall short in sufficiently documenting these vital incidents. Successful management of SAEs in biopharma studies hinges on robust reporting practices, which are essential for informed decision-making and regulatory compliance. By prioritizing SAE monitoring, investigators can ensure a safer environment for participants and foster confidence in the study process.
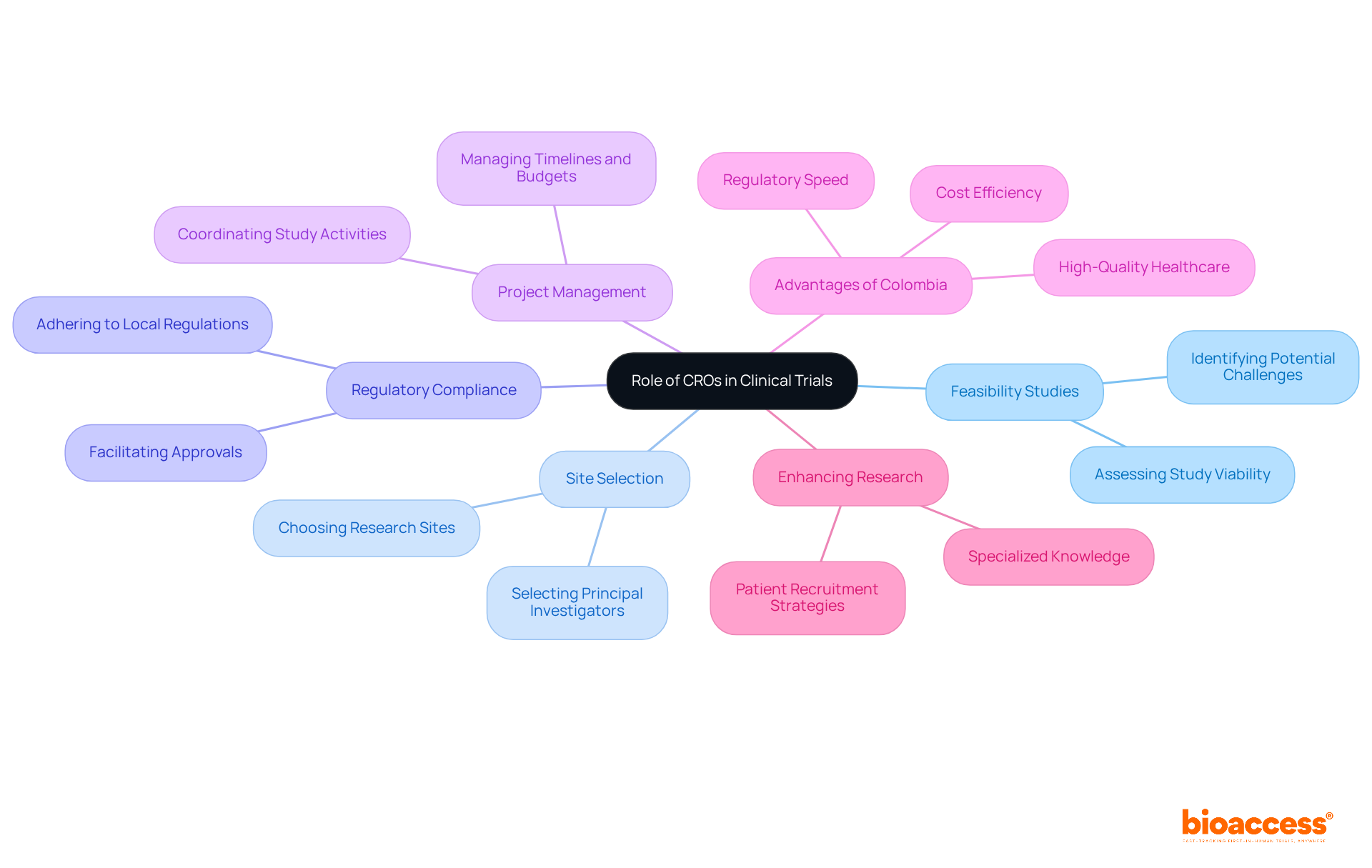
Contract Research Organizations (CROs) are integral to the administration and implementation of medical studies. They offer a comprehensive range of services, including:
By collaborating with CROs such as bioaccess™, sponsors can leverage specialized knowledge to manage the complexities of research studies, ensuring adherence to country-specific regulations while enhancing patient recruitment strategies.
Colombia stands out with its competitive advantages—cost efficiency, regulatory speed, and high-quality healthcare—making it an attractive location for first-in-human research trials. Partnerships like that between bioaccess™ and Caribbean Health Group, supported by the Colombian Minister of Health, further bolster this initiative, positioning Barranquilla as a leading center for medical studies in Latin America. By harnessing the resources and expertise of CROs, sponsors can significantly improve the efficiency and success of their clinical research initiatives.
The exploration of the CDMS medical abbreviation underscores its vital role in enhancing the efficiency and accuracy of clinical research. By integrating advanced technologies and streamlined processes, CDMS solutions, such as those offered by bioaccess®, facilitate rapid patient enrollment and ensure compliance with regulatory standards, ultimately driving successful outcomes in medical studies.
Key insights from the article highlight the significance of various components of clinical data management, including:
Each of these elements contributes to safeguarding data integrity and participant welfare while promoting operational efficiency. Furthermore, the essential roles of Institutional Review Boards (IRBs) and Contract Research Organizations (CROs) are underscored for their contributions to ethical oversight and effective study management.
As the landscape of clinical research continues to evolve, embracing innovative data management practices is crucial for organizations aiming to stay competitive. The integration of CDMS solutions and adherence to established protocols will not only enhance the quality of research but also foster trust among stakeholders. It is imperative for researchers and sponsors to prioritize these best practices to navigate the complexities of modern clinical trials successfully.
What is bioaccess® and what services does it provide?
bioaccess® is a company that offers clinical data management services, leveraging expertise across Latin America, the Balkans, and Australia. It focuses on improving health information management, achieving swift ethical approvals, and enhancing patient enrollment rates for Medtech, Biopharma, and Radiopharma studies.
How quickly can bioaccess® secure ethical approvals?
bioaccess® can secure ethical approvals in an impressive timeframe of 4-6 weeks.
What is the significance of bioaccess®'s patient enrollment rates?
bioaccess® achieves patient enrollment rates that are over 50% higher compared to traditional markets, which significantly improves the efficiency of clinical studies.
Why is effective data management important in clinical research?
Effective data management is crucial due to the increasing complexity of research study endpoints, which have surged by 86% over the past decade. It ensures study success and compliance with regulatory standards.
Can you provide an example of a successful case study involving bioaccess®?
An example is Avantec Vascular's first-in-human trial in Latin America for an innovative vascular device, which showcased bioaccess®'s tailored approach to meeting regulatory requirements and fostering innovation.
How does bioaccess® ensure compliance with regulations?
bioaccess® has established pre-qualified networks with over 50 locations activated in under 8 weeks, ensuring compliance with FDA/EMA/MDR regulations and facilitating expedited patient recruitment and site activation.
What is a Clinical Data Management System (CDMS)?
A Clinical Data Management System (CDMS) is a software application that streamlines the management of information generated during research studies, facilitating data collection, validation, and analysis while ensuring accuracy and regulatory compliance.
What are the benefits of using a CDMS in research studies?
Utilizing a CDMS enhances data integrity, improves efficiency, and ensures compliance with regulatory standards, which is vital for the success of research initiatives.
How does bioaccess® utilize CDMS technology in its services?
bioaccess® offers comprehensive clinical study management services that include feasibility assessments, site selection, compliance evaluations, study setup, import permits, project management, and reporting, leading to faster patient enrollment and cost savings.
What recent advancements have been made in the CDMS landscape?
Recent advancements include innovative platforms like Veeva SiteVault CTMS, which enhance data flow between sites and sponsors, and a trend towards decentralized and hybrid evaluations that rely on real-time data collection.
What is a Case Report Form (CRF) and its role in clinical trials?
A Case Report Form (CRF) is a pivotal document in clinical research that collects essential information from participants, such as demographics, treatment protocols, and outcomes, ensuring the integrity of trial results.
How can CRF design impact clinical research efficiency?
A well-structured CRF can significantly reduce the time needed to complete forms, leading to enhanced overall efficiency in clinical research, with time savings of up to 99.4% compared to traditional methods.
Why is optimal CRF design important?
Optimal CRF design directly influences the quality of analysis and reliability of study outcomes, making it essential for achieving robust and trustworthy results in research studies.