


The primary objective of this article is to delineate the essential components of an investigational brochure (IB) for clinical trials. It underscores the necessity of a meticulously structured IB, which must encompass critical elements such as:
These components are indispensable for safeguarding participant safety, ensuring regulatory compliance, and contributing to the overall success of the clinical trial process.
The investigational brochure (IB) is a cornerstone in the realm of clinical trials, encapsulating vital information that guides researchers and regulatory bodies alike. A well-structured IB possesses the potential to make or break a study; it not only enhances participant safety but also fosters clarity in communication among all stakeholders involved. As the complexities of clinical research continue to evolve, researchers must ask: how can their investigational brochures remain relevant, compliant, and effective? This article delves into the seven key elements that every investigational brochure must include to navigate the challenges of clinical trials successfully.
The investigational brochure (IB) serves as a pivotal document summarizing essential information about the investigational product, encompassing its pharmacological characteristics, preclinical data, and study protocols. This investigational brochure serves as a vital reference for both investigators and regulatory authorities, ensuring alignment on the study's objectives and methodologies.
A meticulously crafted investigational brochure is essential for the effective initiation and execution of research studies, as it lays the groundwork for informed decision-making throughout the research process. Notably, insufficient investigational brochures contribute to approximately 80% of studies facing delays or shutdowns, highlighting the critical need for clarity and thoroughness.
Successful instances of investigational brochures in Medtech clinical studies illustrate that a well-structured investigational brochure can significantly enhance participant safety and compliance, ultimately leading to improved outcomes. As highlighted in recent discussions, it is imperative that the investigational brochure be regularly updated to reflect new findings and maintain its relevance, thereby ensuring it continues to support effective risk assessments and safeguard participant welfare.
The investigational brochure must adhere to regulations established by authorities such as the FDA and EMA. Essential requirements include:
Ensuring that the investigational brochure meets these standards is crucial for securing ethical approvals and maintaining the confidence of participants and stakeholders throughout the study. Moreover, a thorough procedure for advancing medical device evaluations encompasses:
These are vital for addressing regulatory challenges and achieving successful evaluation outcomes. Bioaccess offers expertise in these areas, providing support in:
All of which are essential for navigating the complexities of research studies.
A well-organized investigational brochure is crucial for the success of research trials, serving as a comprehensive resource for all stakeholders. Essential components to include are:
In addition to these elements, it is vital to consider the comprehensive research management services that support the creation of a successful investigational brochure. Services such as feasibility studies, site selection, compliance reviews, testing setup, and project management play a significant role in ensuring that the IB is not only informative but also aligned with regulatory requirements. Typically, successful Investigator's Brochures contain around 80 to 100 pages, balancing detail with conciseness, which is crucial for effective communication. Expert opinions indicate that a well-managed investigational brochure is essential for clear communication in medical research, enhancing the overall integrity of the study process. Furthermore, the investigational brochure must be reviewed and updated at least annually or whenever significant new data is received to ensure its relevance. It is also important to note that the Clinical Trial Application should be submitted along with the investigational brochure to national competent authorities. Examples of comprehensive IBs can be found in various clinical trials, showcasing best practices in documentation and stakeholder engagement. To maximize the effectiveness of the IB, consider leveraging the expertise and services offered by bioaccess, which can streamline the process and ensure compliance with all regulatory requirements.
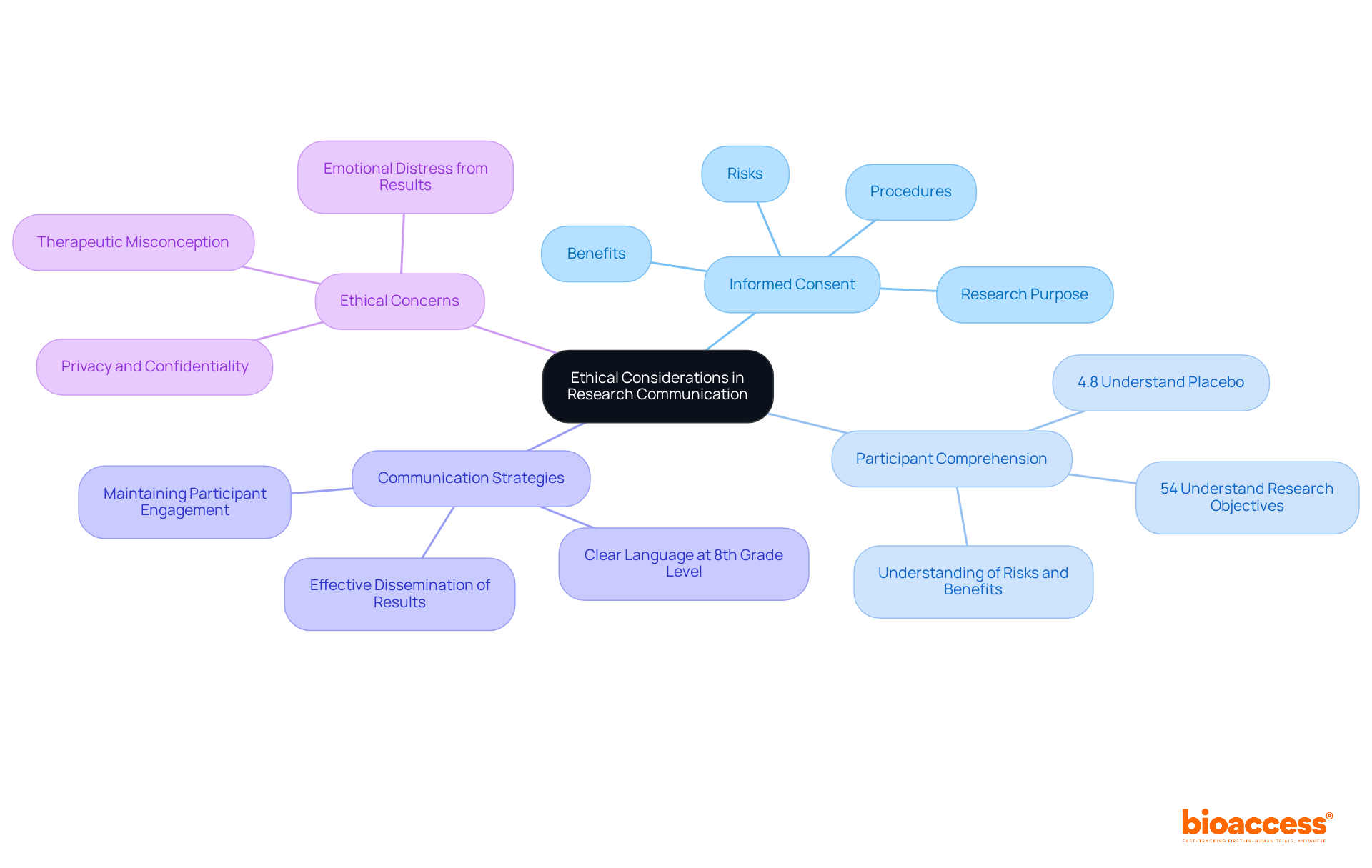
The Investigator's Brochure (IB) must prioritize ethical considerations. It is essential that participants are thoroughly informed about the research's purpose, procedures, risks, and benefits, including their right to withdraw from ancillary research. Effective communication through the investigational brochure is crucial for fostering trust and transparency, which are vital elements for successful participant recruitment and retention. To enhance comprehension, researchers should utilize clear, accessible language in the investigational brochure, ideally at an 8th-grade reading level or lower. This approach not only supports informed consent but also positively influences participant retention rates, as individuals are more likely to remain engaged when they fully understand the implications of their involvement.
Statistics indicate that comprehension levels for informed consent elements can be low, with only 54% of participants grasping research objectives and a mere 4.8% understanding the concept of placebo. Furthermore, maintaining a data file that tracks all signed ancillary consent forms is essential for ethical practices in participant communication. As one researcher noted, "disseminating results without discussion and context can result in more questions...without a process to address these issues, participants may be left with serious concerns without any way to address them.
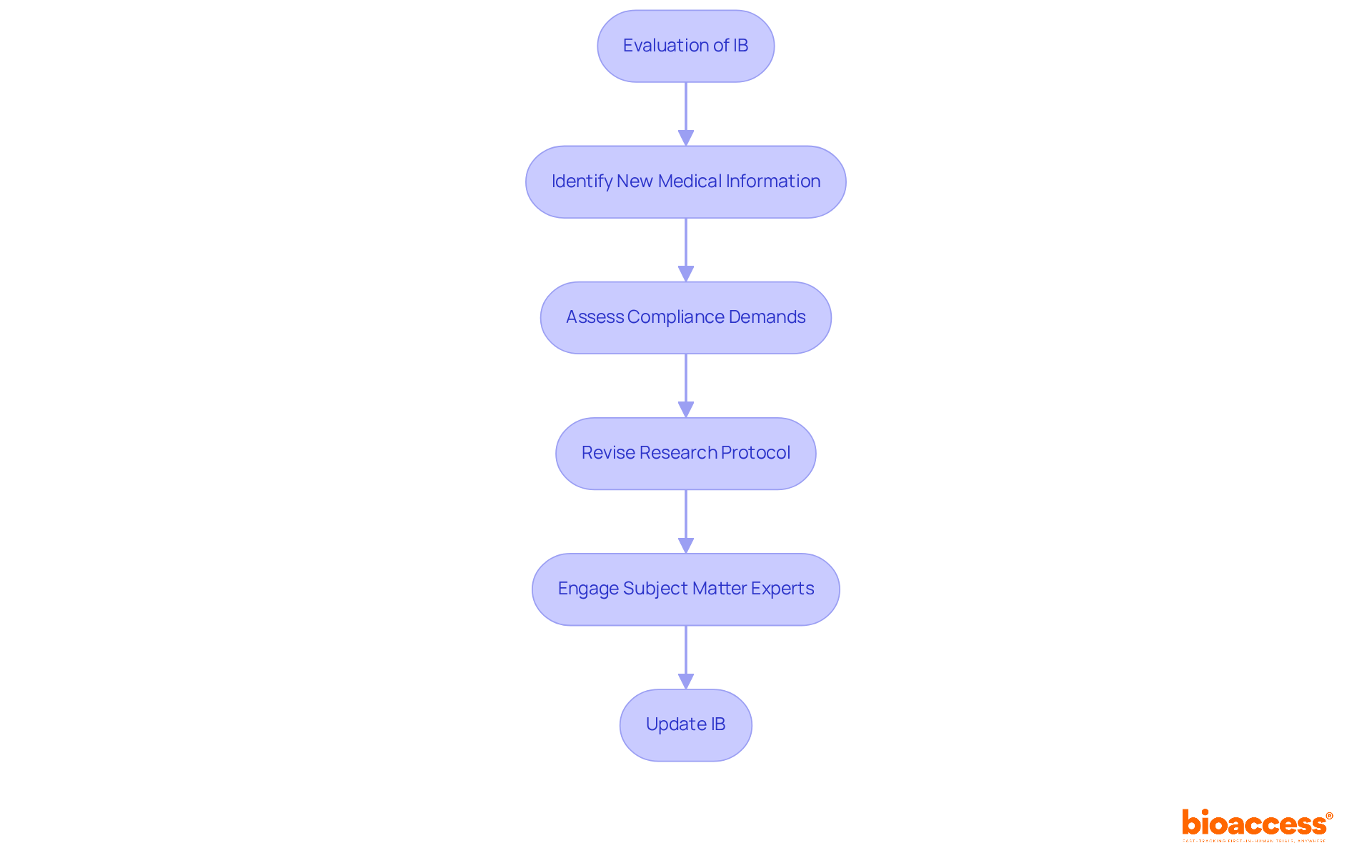
To uphold the integrity of the Investigator's Brochure (IB), a systematic approach to updates and revisions is crucial. Frequent evaluations of the IB in response to new medical information, changing compliance demands, and alterations to the research protocol are essential. Statistics indicate that outdated IBs can significantly compromise participant safety, underscoring the necessity of timely updates.
For instance, maintaining an accurate IB can enhance the success rates of clinical trials, with Phase III completion rates rising to 66% when local compliance knowledge is utilized. Moreover, professional perspectives emphasize that a well-maintained investigational brochure not only ensures adherence but also safeguards participant welfare by providing the latest information regarding the investigational product and protocols.
Engaging subject matter experts early in the revision process can streamline updates and mitigate potential risks, reinforcing the commitment to participant safety and research integrity.
Preparing the investigational brochure for regulatory review demands meticulous attention to detail and strict adherence to established guidelines. It is crucial to confirm that the investigational brochure is comprehensive, incorporating essential elements such as:
Additionally, supporting documents, including the research protocol and informed consent forms, must be compiled and organized effectively. Engaging in feasibility studies and selecting qualified research sites and principal investigators (PIs) is vital at this stage to ensure compliance with country-specific requirements. Moreover, considering the trial arrangement, import authorizations, and project oversight is essential to facilitate a seamless compliance process.
Submitting a complete and well-organized package to the relevant oversight authority is imperative, as it can significantly reduce turnaround times. Statistics reveal that applications receiving more substantial oversight had median turnaround times of:
This underscores the importance of thorough preparation and adherence to legal standards to enable timely approval for clinical trials, including acquiring necessary approvals from ethics committees and health ministries. Regular updates to the investigational brochure are equally essential to reflect any changes in the investigational product's safety profile and recent research findings, ensuring that the document remains relevant and compliant.

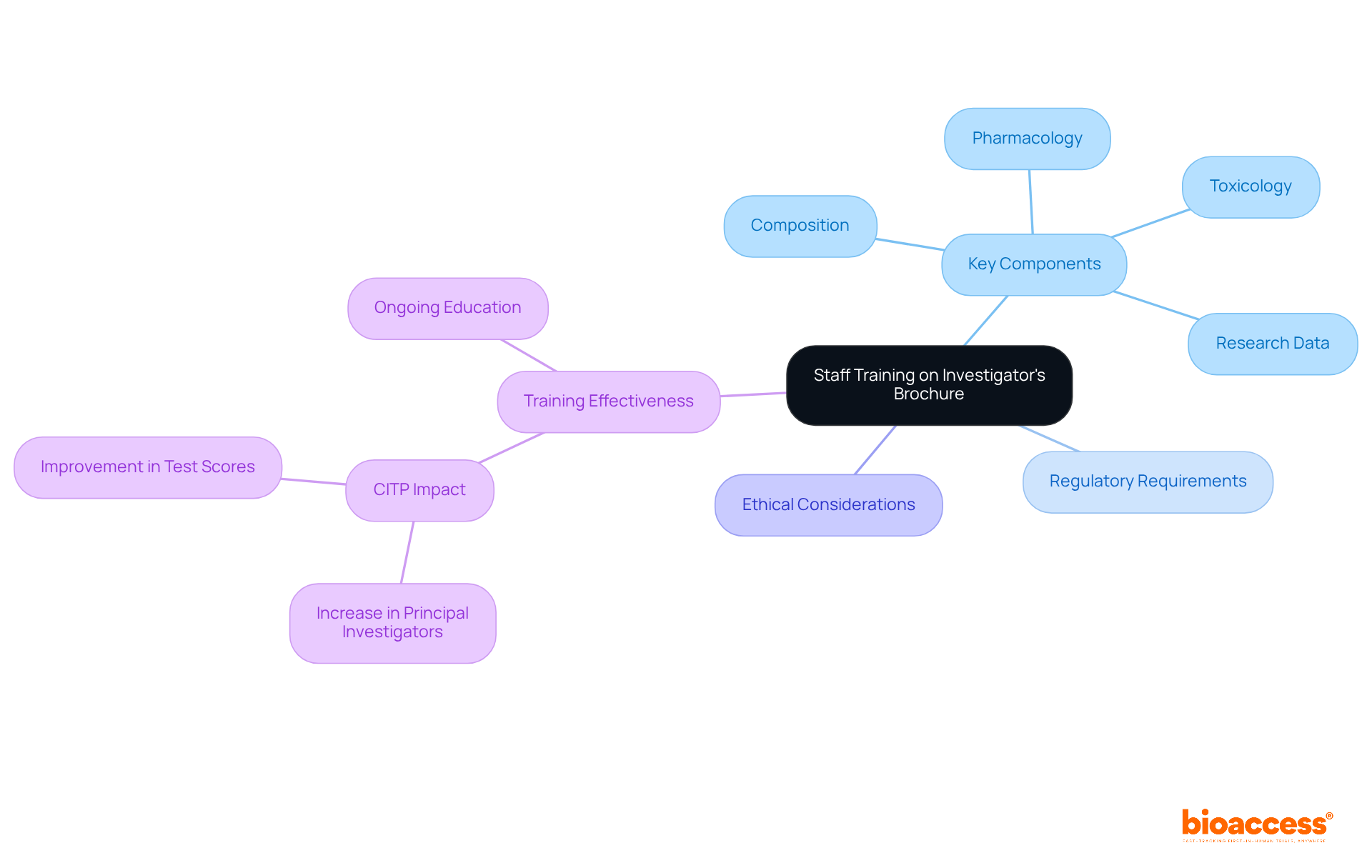
Educating researchers about the investigational brochure is essential for ensuring that all team members grasp its contents and significance. Training sessions must comprehensively cover the key components of the investigational brochure, along with regulatory requirements and ethical considerations. Studies indicate that 60% of researchers express confidence in their understanding of the IB, underscoring the need for effective training programs. Ongoing education is vital to keep staff updated on revisions and new information related to the investigational brochure, as it should be regularly updated to reflect changes in the investigational product's safety profile and recent research findings.
By promoting a culture of knowledge and adherence, research groups can significantly improve the quality and integrity of studies, ultimately resulting in enhanced patient safety and study effectiveness. As Sarah Lee observed, 'The investigational brochure is an essential document that describes the details of the investigational product, including its composition, pharmacology, toxicology, and research data.'
Furthermore, the number of participants achieving first-time principal investigator status increased from 17 to 33 following the Clinical Investigator Training Program (CITP), illustrating the effectiveness of training programs. The investigational brochure should also be shared with all relevant stakeholders, ensuring that everyone involved in the process has access to the most current information.
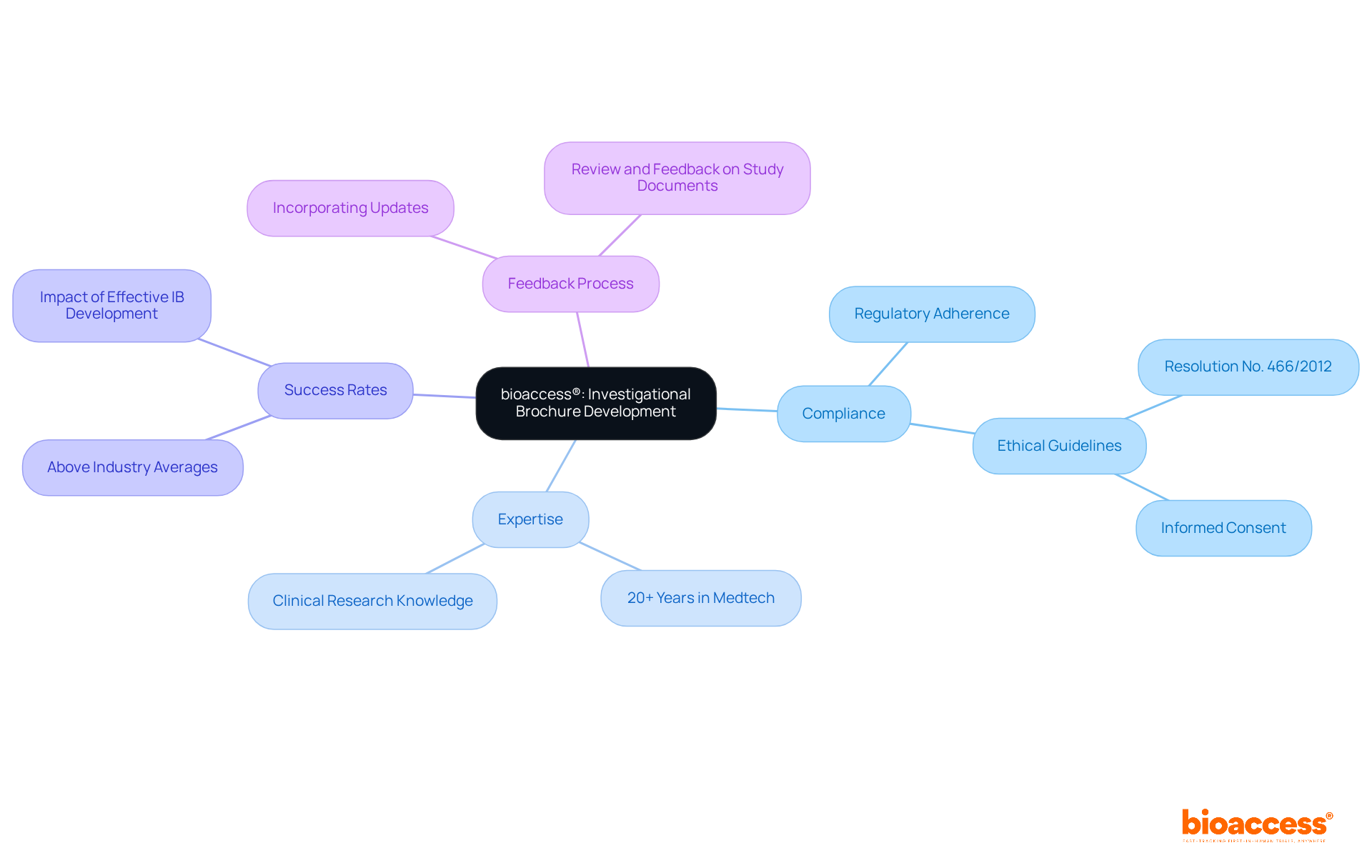
bioaccess® excels in enhancing the creation of investigational brochures for research studies, ensuring that all essential components are meticulously included. Our group of experienced experts is committed to upholding compliance with regulations and tackling ethical issues, which are essential for the successful execution of the study.
With more than 20 years of experience in the Medtech field, we utilize our extensive knowledge of the clinical research environment to create high-quality investigational brochures that not only assist in gaining approval but also enhance the overall success rates of clinical studies. Research shows that effective IB development can greatly enhance testing results, with success rates for tests facilitated by bioaccess® exceeding industry averages.
By incorporating comprehensive details and adhering to the latest regulatory standards, including guidelines outlined in Resolution No. 466/2012, we enable researchers to navigate the complexities of clinical studies with confidence. Our commitment to excellence in the creation of the investigational brochure is further underscored by our proactive approach to integrating feedback and updates, ensuring that each brochure remains relevant and aligned with evolving research needs.
Additionally, we provide thorough review and feedback on study documents to comply with country requirements, enhancing the overall quality of the trial process.
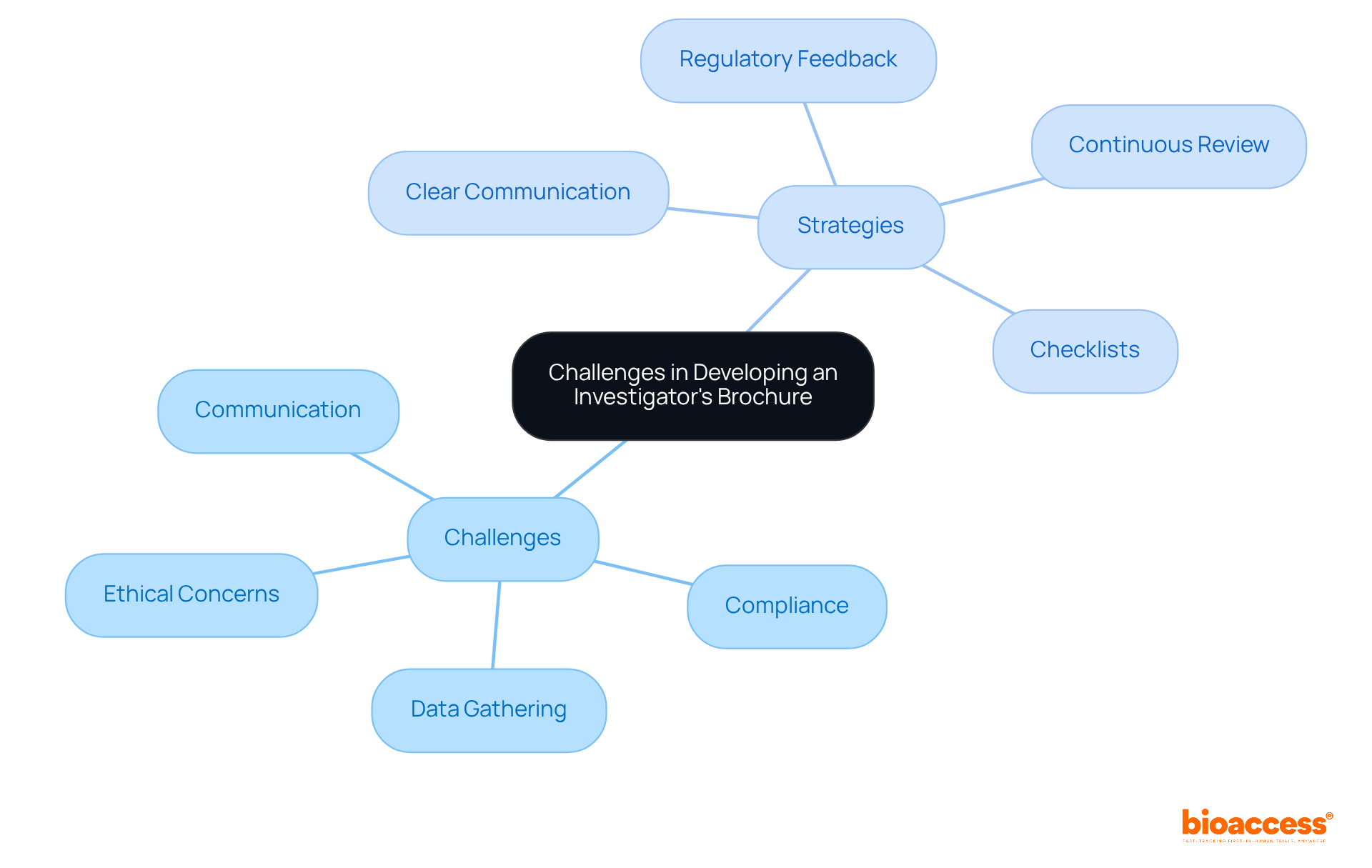
Creating an investigational brochure presents various challenges, including the necessity for thorough data gathering, compliance with standards, and the handling of ethical concerns. A significant hurdle lies in effectively communicating complex information in a manner accessible to all stakeholders. Notably, research indicates that 63% of participants are familiar with the CTFG Q&A document; however, many still find the summary of data and guidance for the investigator (Section 7) in need of improvement.
To navigate these challenges, establishing clear communication channels among team members is crucial. Engaging regulatory experts for feedback can enhance the quality of the investigational brochure, as evidenced by 55% of participants rating the content quality as 4 out of 5. Continuous review and revision of the investigational brochure is essential for maintaining its relevance and accuracy as new information emerges.
Strategies such as utilizing comprehensive checklists can significantly improve documentation quality, ensuring that all required elements are included and enhancing compliance. By proactively addressing these typical obstacles, researchers can create a well-organized investigational brochure that facilitates the effective execution of research studies.
Engaging sponsors and investigators in the development of the investigational brochure (IB) is essential for capturing diverse perspectives and ensuring comprehensive documentation. This collaboration fosters a more robust IB that effectively addresses the needs and concerns of all stakeholders involved.
To facilitate this collaboration, regular meetings and open communication channels are vital. These interactions not only allow for the exchange of ideas but also enable constructive feedback, ultimately enhancing the quality and effectiveness of the IB.
By actively involving sponsors and investigators in the IB process, organizations can significantly improve the clarity and relevance of the information presented, leading to better compliance and outcomes in clinical trials.
The investigational brochure (IB) is an essential document that plays a pivotal role in the success of clinical trials. It serves as a comprehensive resource, outlining the investigational product and its associated risks while ensuring that all stakeholders are informed and aligned throughout the research process. The significance of a well-constructed IB cannot be overstated, as it directly impacts participant safety, regulatory compliance, and the overall integrity of the clinical study.
Key elements of an effective investigational brochure have been explored, including:
By adhering to established guidelines and fostering collaboration among sponsors and investigators, researchers can create a robust IB that enhances communication and facilitates a smoother approval process. Evidence highlights that a meticulously crafted IB not only aids in meeting regulatory requirements but also contributes to higher success rates in clinical trials.
In light of these insights, it is imperative for researchers and organizations involved in clinical trials to prioritize the development and maintenance of the investigational brochure. By investing time and resources into creating a high-quality IB, stakeholders can ensure compliance with regulatory standards and demonstrate their commitment to safeguarding participant welfare. Embracing best practices in IB development will ultimately lead to more effective clinical trials, improved outcomes, and a stronger foundation for future medical advancements.
What is an Investigator's Brochure (IB)?
The Investigator's Brochure is a pivotal document that summarizes essential information about an investigational product, including its pharmacological characteristics, preclinical data, and study protocols. It serves as a vital reference for investigators and regulatory authorities.
Why is a well-crafted Investigator's Brochure important?
A meticulously crafted Investigator's Brochure is essential for the effective initiation and execution of research studies. It lays the groundwork for informed decision-making and helps prevent delays or shutdowns in studies, as insufficient brochures contribute to approximately 80% of such issues.
What are the key regulatory compliance requirements for an Investigator's Brochure?
The Investigator's Brochure must adhere to regulations established by authorities like the FDA and EMA, which include a clear description of the investigational product, detailed information on the research design, and a comprehensive risk evaluation.
What essential components should be included in an Investigator's Brochure?
Essential components include:
How often should the Investigator's Brochure be updated?
The Investigator's Brochure should be reviewed and updated at least annually or whenever significant new data is received to ensure its relevance.
What role do comprehensive research management services play in creating an Investigator's Brochure?
Comprehensive research management services, such as feasibility studies, site selection, compliance reviews, testing setup, and project management, support the creation of a successful Investigator's Brochure and ensure alignment with regulatory requirements.
What is the typical length of a successful Investigator's Brochure?
Successful Investigator's Brochures typically contain around 80 to 100 pages, balancing detail with conciseness for effective communication.
What should be submitted alongside the Investigator's Brochure to regulatory authorities?
The Clinical Trial Application should be submitted along with the Investigator's Brochure to national competent authorities.
How can Bioaccess assist with the Investigator's Brochure process?
Bioaccess offers expertise in study setup, import permits, project management, and reporting, which are essential for navigating the complexities of research studies and ensuring compliance with regulatory requirements.