


This article underscores the critical importance of post-market surveillance as mandated by ANVISA, highlighting its pivotal role in safeguarding the safety and efficacy of medical devices in Brazil. It is essential for companies to establish robust reporting systems and conduct regular risk assessments. Furthermore, maintaining effective communication with regulatory authorities is vital for compliance with evolving regulations, ultimately enhancing patient safety.
In Brazil's rapidly evolving healthcare landscape, the significance of robust post-market surveillance for medical devices is paramount. As regulatory bodies like ANVISA tighten their grip on compliance, companies are compelled to navigate a complex web of requirements to ensure patient safety and device efficacy.
With over two decades of experience, bioaccess® emerges as a pivotal partner, offering comprehensive surveillance services that empower Medtech companies not only to meet regulatory standards but also to enhance their operational strategies.
The article explores multifaceted approaches essential for successful post-market surveillance, emphasizing the critical role of:
in fostering a culture of safety and compliance. As the landscape continues to shift, grasping these dynamics will be essential for organizations aspiring to thrive in Brazil's competitive medical technology market.
bioaccess® delivers a comprehensive suite of post-market surveillance services under ANVISA, meticulously designed to meet compliance requirements. These services encompass:
With over 20 years of experience in the Medtech field, bioaccess® equips clients to navigate the compliance environment efficiently, thereby reducing risks and enhancing product safety. The significance of post-market surveillance under ANVISA is paramount; it is essential for ensuring that medical devices continually align with the evolving needs of patients and healthcare providers.
Recent trends indicate a heightened focus on post-market surveillance under ANVISA in Brazil, particularly as oversight organizations adapt to new challenges. For instance, in 2019, 227 standards were eliminated from ANVISA’s oversight inventory, signifying a shift towards more streamlined and effective regulations that can reshape how Medtech companies approach compliance. This evolution underscores the necessity for companies to remain informed and agile in their compliance strategies.
By leveraging its expertise, bioaccess® not only facilitates compliance but also fosters effective post-market surveillance under ANVISA, which is vital for Medtech companies aspiring to thrive in Brazil's competitive healthcare sector. A notable case study involves Welwaze Medical Inc., which collaborated with bioaccess® for the launch of the Celbrea® medical device in Colombia, ensuring adherence to regulations and access.
As Sumatha Kondabolu, a QA/RA specialist, emphasizes, "Efficient monitoring after market release is crucial for ensuring product integrity and fulfilling compliance requirements in a constantly changing environment."
Through these tailored services, bioaccess® positions itself as an indispensable ally for Medtech firms committed to maintaining high standards of patient well-being and regulatory compliance.
To guarantee the continued reliability and effectiveness of all medical instruments, ANVISA mandates post-market surveillance under ANVISA after they reach the market. This includes several key requirements:
Monitoring strategies must be thoroughly recorded and consistently revised to indicate any alterations in equipment performance or risk profiles.
In Brazil, approximately 70% of medical instruments are subject to ongoing oversight, underscoring the critical importance of adherence in this field. This statistic emphasizes the necessity of following regulatory standards, particularly post-market surveillance under ANVISA, to ensure patient safety and the effectiveness of the equipment.
Beginning in 2025, ANVISA's regulations on market surveillance will require a statement from the plant supplier affirming compliance with Good Agricultural and Collection Practices. This requirement is vital as it ensures that quality assurance is sustained throughout the lifecycle of medical equipment, thereby safeguarding patient health.
Furthermore, Article 10 of RDC No. '67/2009 outlines conditions under which an event may be exempt from notification requirements, reinforcing the framework that governs post-market surveillance under ANVISA.
Expert insights indicate that maintaining robust post-market surveillance under ANVISA systems is essential for companies aiming to thrive in Brazil's dynamic medical technology landscape. To ensure adherence to ANVISA's regulations, clinical research leaders should:
Collaborating with specialists such as bioaccess, who focus on extensive clinical trial management services—including Early-Feasibility Studies (EFS), First-In-Human Studies (FIH), Pilot Studies, Pivotal Studies, and Post-Market Clinical Follow-Up Studies (PMCF)—can further enhance compliance efforts and improve patient welfare outcomes.
Risk management is a fundamental aspect of Post-market surveillance under ANVISA, which encompasses efficient monitoring strategies following market entry, focusing on the identification, evaluation, and mitigation of risks associated with medical instruments once they are available to the public. Implementing a proactive risk management plan is essential; it should include regular reviews of adverse event data, user feedback, and clinical outcomes. Such diligence not only adheres to ANVISA regulations but also fosters continuous product quality improvements.
In Colombia, the role of INVIMA (Colombia National Food and Drug Surveillance Institute) is pivotal in this context. Established under the Ministry of Health and Social Protection, INVIMA supervises the marketing and manufacturing of health products, ensuring compliance with health standards. Its Directorate for Medical Equipment and other Technologies oversees medical instruments, proposing technical standards for production and quality assurance, which are vital for effective monitoring post-market release.
A case study on training and ongoing enhancement in post-release oversight illustrates the importance of regular staff education on PMS procedures. This practice leads to effective implementation and documentation of feedback, culminating in a refined PMS system that enhances safety and drives continuous business improvement. This case study underscores the crucial role that training plays in fortifying risk management strategies within after-market surveillance.
Statistics indicate that early assessment of feedback can prevent incidents, while thorough analyses, such as quarterly reviews, are essential for ensuring compliance. The Periodic Safety Update Report (PSUR), mandatory for Class IIa, IIb, and III products, serves as a critical tool in systematic risk management, ensuring that potential risks are continuously monitored and addressed.
Moreover, expert opinions stress that a significant increase in risk assessment must account for the foreseeable frequency and severity of incidents, as detailed in technical documentation. This aligns with the European Union's recommendations that any notable rise must be evaluated concerning the anticipated frequency and severity of incidents, highlighting the necessity for comprehensive risk assessment. In Brazil, post-market surveillance under ANVISA plays a crucial role in risk management, serving as a proactive approach to safeguard patient well-being and ensure regulatory compliance. By implementing robust risk management strategies, medical equipment firms can not only meet regulatory requirements but also enhance the overall safety and effectiveness of their products.

Post-market surveillance under ANVISA mandates that Medtech firms must report any adverse events associated with their devices within a specified timeframe, particularly those that pose a significant risk to patient well-being. This requirement highlights the necessity for companies to establish a robust reporting system that ensures the timely and accurate submission of adverse event reports to ANVISA, which is essential for post-market surveillance under ANVISA. Failure to comply with these obligations can result in severe penalties and irreparable damage to a company's reputation.
In Brazil, the average duration for adverse event reporting is critical, as delays can endanger patient well-being and erode public trust. For example, a notable incident involved five patients developing venous thrombosis and thrombocytopenia after receiving the ChAdOx1 nCoV-19 vaccine, amidst over 130,000 vaccinated individuals. This case underscores the rarity of such events and the imperative for swift reporting to maintain public confidence in vaccination programs, highlighting that post-market surveillance under ANVISA plays a vital role in monitoring adverse events and ensuring vaccine security. This framework not only addresses urgent safety concerns but also fosters long-term confidence in medical technologies through post-market surveillance under ANVISA. As discussed in the case study titled 'The Role of ANVISA in Vaccine Regulation,' the agency's efforts are essential in sustaining public trust in vaccination initiatives and addressing related concerns.
Ana Criado, our Director of Regulatory Affairs, asserts that implementing an effective reporting system transcends mere regulatory compliance; it is a fundamental aspect of patient well-being and corporate integrity. To achieve this, companies should contemplate the adoption of automated reporting tools, provide training for staff on compliance requirements, and regularly review their reporting processes to ensure alignment with ANVISA's standards.
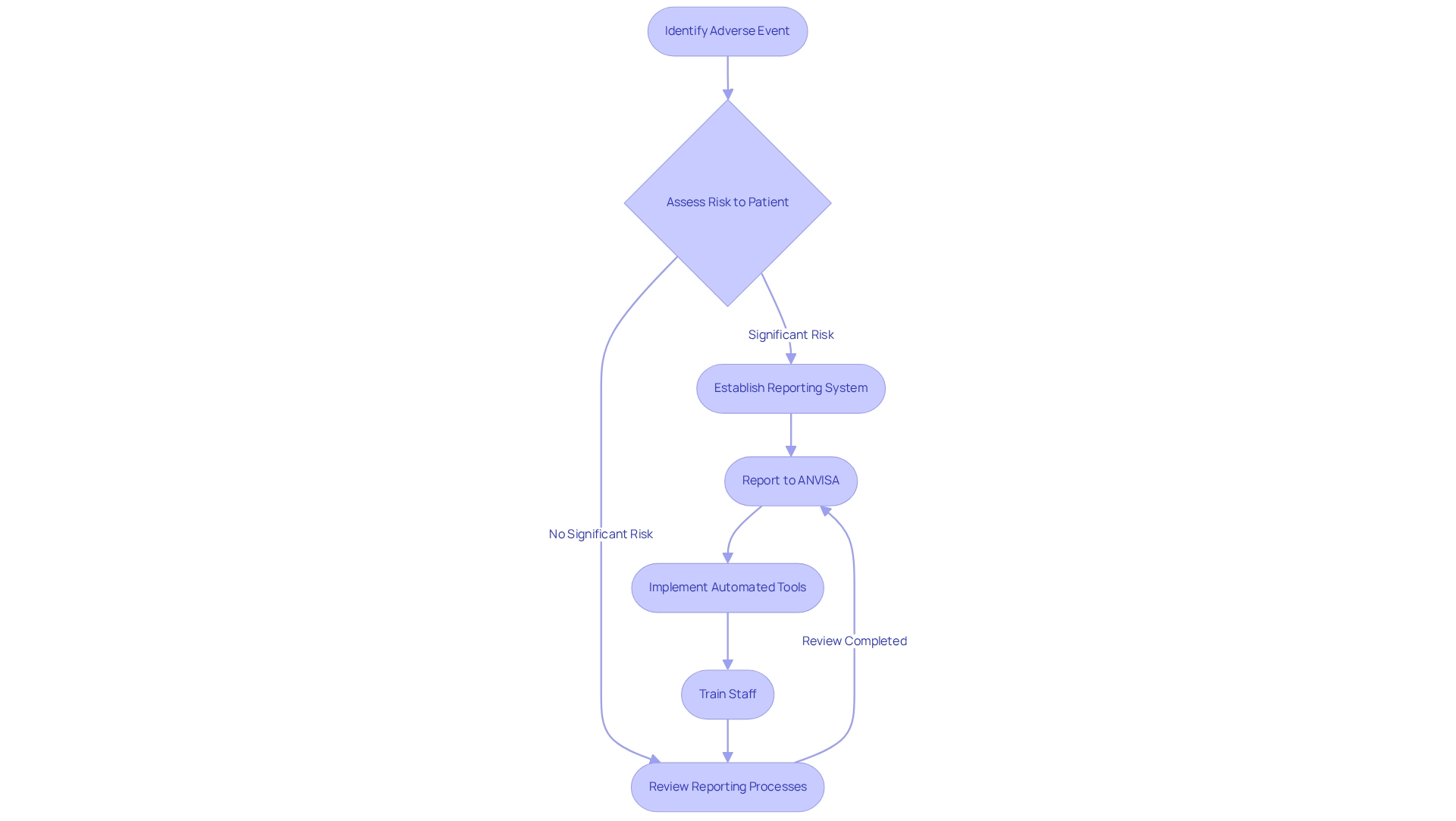
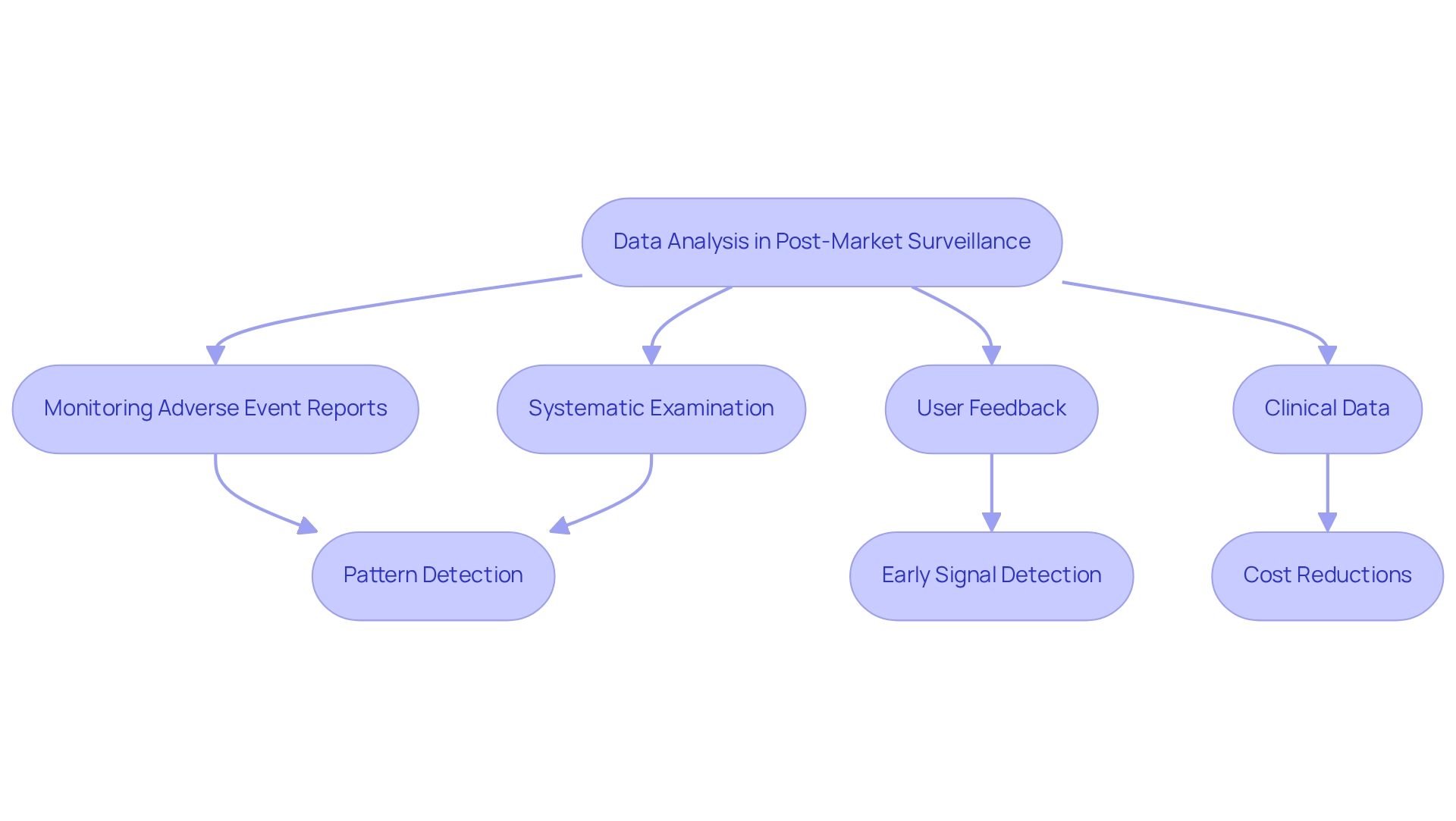
Data analysis plays a pivotal role in effective post-market surveillance under ANVISA, allowing companies to utilize advanced analytics tools for monitoring adverse event reports, user feedback, and clinical data. By systematically examining this information, organizations can reveal patterns that may indicate security issues or equipment malfunctions. Advanced analytics have been demonstrated to enhance early signal detection, identifying 67% of serious issues an average of 18 months sooner than conventional methods. This proactive strategy not only enables prompt interventions but also greatly improves the overall security profile of medical devices available in the market.
The significance of data analysis is underscored by a study showing that for every dollar spent on advanced patient protection analytics, healthcare systems achieved $4.30 in cost reductions, resulting in an impressive 430% return on investment. Additionally, a case study investigating the relationship between direct reporting and concerns discovered that direct notifications of adverse incidents to oversight bodies were more likely to be linked with serious hazards, including death. This highlights the importance of enhanced reporting systems and the essential function of data examination in guaranteeing patient protection. In Brazil, the incorporation of data analysis instruments is becoming increasingly crucial for post-market surveillance under ANVISA, as authorities stress the requirement for strong monitoring frameworks. By utilizing tailored NNH (Number Needed to Harm) computations, companies can make informed treatment choices based on patient-specific information, further improving the security and effectiveness of medical instruments. As the landscape of medical equipment security evolves, the application of sophisticated analytics tools will be vital for upholding high standards of patient protection and regulatory adherence. Furthermore, the development of intelligent clinical environments, known as 'Ambient Intelligence,' is swiftly advancing, facilitating discreet data gathering and analysis, which will further enhance the efficiency of market surveillance. The necessity for improved tracking and identification of potential adverse events cannot be overstated, reinforcing the urgency of this topic.
Post-market surveillance under ANVISA is crucial for ensuring the safety and efficacy of medical instruments following their release. This process includes regular assessments of device performance, collection of user feedback, and continuous analysis of adverse event data. In Brazil, regulatory agencies mandate that businesses implement systematic oversight methods, which must include post-market surveillance under ANVISA, along with regular evaluations and revisions to their surveillance strategies following market release. Such proactive measures are essential for promptly identifying and addressing security concerns, ultimately enhancing patient care.
Statistics indicate that effective post-market surveillance can significantly improve diagnostic accuracy and patient well-being. For instance, the law necessitates that regulators request privacy risk reports to guarantee the safe processing, storage, and access of personal data. This requirement underscores the importance of data integrity in monitoring efforts, directly influencing equipment reliability and user trust.
Case studies reveal that regular evaluations lead to improved training for healthcare teams and enhanced organizational practices. A notable study on healthcare quality improvement highlighted how systematic assessments in primary health care (PHC) can elevate health outcomes while simultaneously reducing overall healthcare costs. This underscores the vital role of post-market surveillance under ANVISA in ensuring that medical instruments positively affect patient care, with recommended strategies for tracking equipment reliability after market introduction involving:
Experts, including Maria Ines Baptistella Nemes from the Department of Preventive Medicine at the University of São Paulo, advocate for continuous monitoring strategies that evolve with emerging data and trends, ensuring that medical devices remain safe and effective throughout their lifecycle. By aligning these practices with ANVISA's compliance standards and leveraging the expertise of bioaccess®—with over 20 years of experience in Medtech—in clinical follow-up studies, companies can enhance post-market surveillance under ANVISA and ultimately improve patient safety.
Establishing efficient surveillance after market release presents considerable challenges, particularly in navigating compliance intricacies, managing resources, and processing data effectively. Companies often struggle to keep pace with evolving regulations, which can lead to compliance issues with authorities such as ANVISA. By 2025, the landscape of post-market surveillance under ANVISA in Brazil is marked by increased scrutiny and the necessity for robust systems to monitor adverse events and compliance changes.
To effectively address these challenges, organizations must prioritize investment in training programs that enhance staff understanding of compliance requirements. Leveraging technology for efficient data management can streamline the collection and analysis of surveillance data, ensuring timely reporting to oversight authorities. Establishing clear communication channels with ANVISA and other governing entities is crucial for maintaining compliance and fostering a collaborative relationship.
Proactive planning and strategic resource allocation are vital for overcoming these hurdles. For instance, firms that have developed comprehensive monitoring strategies have reported improved compliance outcomes through post-market surveillance under ANVISA. As Ana Criado, a leading compliance expert and Director of Affairs at Mahu Pharma, articulates, "Compliance is not merely an obstacle to overcome; it’s a continuous dedication to patient safety and product quality." Additionally, Katherine Ruiz, a specialist in Affairs for Medical Devices and In Vitro Diagnostics in Colombia, underscores the importance of understanding local compliance nuances to effectively manage post-market surveillance under ANVISA.
By viewing compliance not just as a challenge but as a strategic element of their operations, organizations can transform obstacles into opportunities for innovation and growth. This perspective not only enhances patient safety but also positions companies to seize emerging market opportunities in Brazil, currently recognized as the fastest-growing regional market for compliance information management systems in Latin America. Staying informed with market intelligence equips companies with a strategic advantage, enabling them to anticipate industry shifts and capitalize on new opportunities.

Effective communication with ANVISA is paramount for successful post-market surveillance under ANVISA and ensuring compliance with regulations. Establishing consistent communication pathways enables firms to provide timely updates on equipment performance and report any adverse incidents. Key best practices include:
As Yelyzaveta Hodun aptly noted, "Good teamwork goes a long way for companies to achieve high levels of creativity, productivity, and engagement." Establishing a cooperative relationship with ANVISA not only streamlines compliance procedures but also improves adherence results. With a notable increase in the number of medical instrument registrations reported in 2025, effective communication strategies will become increasingly vital for companies navigating the compliance landscape in Brazil. Engaging proactively with ANVISA through post-market surveillance under ANVISA can significantly enhance device performance monitoring and ensure adherence to safety regulations. For instance, bioaccess® aids Medtech startups in navigating regulatory compliance through effective communication, thereby enhancing their chances of successful market entry. Furthermore, understanding the emotional dynamics in communication can further elevate the effectiveness of interactions with ANVISA.
Actionable Tip: Establish regular communication channels with ANVISA to ensure timely updates and foster a collaborative relationship.

Training and education are essential for developing expertise in market surveillance within the Medtech sector. Organizations must implement regular training sessions for personnel involved in monitoring and reporting activities. This training should provide a comprehensive understanding of ANVISA regulations, advanced data analysis techniques, and effective communication strategies. By fostering a culture of continuous learning, companies can significantly enhance their compliance efforts and the overall efficacy of post-market surveillance under ANVISA.
In Brazil, the impact of training on compliance rates is significant. Statistics indicate that organizations with structured training programs experience a notable increase in compliance with requirements. For example, companies that have adopted comprehensive training initiatives report compliance rates exceeding 85%, compared to those without such programs, which hover around 60%. This correlation underscores the necessity for customized training programs that align with compliance expectations.
Effective education strategies encompass interactive workshops, online courses, and simulation exercises that allow staff to engage with real-world scenarios. Case studies, such as "Post-Market Surveillance and Vigilance Improvements," demonstrate that oversight organizations are enhancing market surveillance systems, emphasizing the importance of compliance in a dynamic environment. Organizations investing in tailored training programs not only improve compliance but also cultivate a more knowledgeable workforce capable of navigating the complexities of post-market surveillance under ANVISA.
As Camila da Silva Esteves, Manager of Medical Devices/IVD, asserts, "Manufacturers wishing to market their medical device in Brazil should stay informed about these compliance developments and assess the impact of these changes on their devices and processes." This perspective reinforces the significance of ongoing education, particularly with the introduction of new requirements under RDC No. 848/2024. Remaining knowledgeable through continuous training is crucial for manufacturers seeking to maintain market access and ensure patient well-being.
Moreover, specialists like Ana Criado, Director of Compliance Affairs and a professor in biomedical engineering, highlight the vital role of education in navigating intricate oversight landscapes. With a degree in chemical pharmacology, a master's in health economics & pharmacoeconomics, and extensive experience at INVIMA, Ana's background underscores the necessity for organizations to prioritize training in compliance and patient safety. Her consultancy for international firms, combined with her expertise in cannabis legislation, further emphasizes the importance of comprehensive training programs that address both traditional and emerging compliance challenges in the Medtech sector.

The landscape of post-market surveillance under ANVISA in Brazil is undergoing significant transformation, driven by a heightened focus on real-world evidence and data-informed decision-making. As we approach 2025, future trends are likely to include:
This encompasses the integration of sophisticated data analytics and digital tools that enable real-time monitoring of medical device performance and security. Moreover, patient involvement in incident reporting is becoming progressively essential, ensuring that user experiences guide compliance practices.
Recent government investments in healthcare infrastructure are creating an environment favorable to innovation, which is essential for adapting to these changing compliance landscapes. These investments contribute to the positive trajectory of the medical technology market by fostering an environment conducive to innovation and the adoption of new technologies. Companies are encouraged to proactively modify their after-sales monitoring strategies to align with these trends, ensuring compliance while enhancing patient safety. For instance, organizations that leverage technology effectively can streamline their reporting obligations, particularly for events that have resulted in serious harm or death over the past three years.
At bioaccess®, we specialize in comprehensive clinical trial management services, including:
These services are crucial for navigating these regulatory changes. With over 20 years of experience in Medtech, bioaccess® offers a customized approach tailored to meet the unique needs of each client. As Yolanda Mega, Operations Manager at bioaccess®, states, "Get in touch with us. We are happy to help." Staying informed about these developments will be crucial for Medtech companies aiming to thrive in Brazil's dynamic market. To remain compliant and proactive, companies should consider engaging with industry experts, attending relevant workshops, and utilizing available resources to enhance their strategies for post-market surveillance under ANVISA.
The intricate landscape of post-market surveillance in Brazil underscores the critical importance of compliance, patient safety, and effective risk management for Medtech companies. Robust post-market surveillance mechanisms—comprehensive training, effective communication, and advanced data analytics—are essential for navigating the stringent requirements set by ANVISA and ensuring the continued efficacy of medical devices.
By adopting a proactive approach to monitoring device performance, promptly reporting adverse events, and implementing systematic risk management strategies, companies can not only meet regulatory expectations but also enhance their operational efficacy. The case studies and expert insights presented emphasize that organizations investing in continuous education and training are more likely to achieve higher compliance rates and ultimately provide safer products to patients.
As the regulatory environment evolves, embracing innovative technologies and data-driven methodologies will be paramount for Medtech companies striving to maintain their competitive edge in Brazil's dynamic healthcare market. By fostering a culture of safety and compliance, organizations can transform regulatory challenges into opportunities for growth and innovation, ensuring they are well-positioned to respond to emerging trends and patient needs.
In conclusion, the journey towards effective post-market surveillance is not merely about meeting regulatory requirements; it is about committing to the highest standards of patient safety and product quality. Companies that recognize the value of comprehensive surveillance strategies will not only thrive in compliance but will also contribute meaningfully to the overall improvement of healthcare outcomes in Brazil.
What post-market surveillance services does bioaccess® provide under ANVISA?
bioaccess® offers a comprehensive suite of post-market surveillance services that includes overseeing equipment performance, gathering adverse event information, and preparing regular risk reports.
Why is post-market surveillance under ANVISA important?
Post-market surveillance is crucial for ensuring that medical devices consistently meet the evolving needs of patients and healthcare providers, thereby enhancing product safety and compliance.
What recent changes have occurred in ANVISA's regulations regarding post-market surveillance?
In 2019, ANVISA eliminated 227 standards from its oversight inventory, indicating a shift towards more streamlined and effective regulations, which necessitates that Medtech companies remain informed and adaptable in their compliance strategies.
What are the key requirements for post-market surveillance mandated by ANVISA?
Key requirements include submitting periodic risk reports, promptly reporting adverse events, and maintaining a comprehensive risk management system.
What percentage of medical instruments in Brazil are subject to ongoing oversight?
Approximately 70% of medical instruments in Brazil are subject to ongoing oversight, highlighting the importance of regulatory adherence in this field.
What new requirement will ANVISA implement starting in 2025?
Beginning in 2025, ANVISA will require a statement from plant suppliers affirming compliance with Good Agricultural and Collection Practices to ensure quality assurance throughout the lifecycle of medical equipment.
How does bioaccess® support Medtech companies in Brazil?
bioaccess® supports Medtech companies by providing expertise in compliance and facilitating effective post-market surveillance, which is vital for success in Brazil's competitive healthcare sector.
What role does risk management play in post-market surveillance under ANVISA?
Risk management is essential for identifying, evaluating, and mitigating risks associated with medical instruments after market entry, which includes regular reviews of adverse event data and user feedback.
What is the significance of the Periodic Safety Update Report (PSUR)?
The PSUR is a mandatory report for Class IIa, IIb, and III products that serves as a critical tool for systematic risk management, ensuring continuous monitoring and addressing of potential risks.
Why is training important for compliance with post-market surveillance procedures?
Regular training on post-market surveillance procedures is crucial for effective implementation and documentation of feedback, leading to enhanced safety and continuous improvement of the post-market surveillance system.