


The article examines pivotal medical device regulatory trends in Latin America for 2025, underscoring the critical nature of:
These trends are vital as they enable manufacturers to achieve faster market entry and compliance, ultimately fostering the growth of the LATAM healthcare equipment industry, projected to reach USD 37.23 billion by 2025. This growth is supported by initiatives such as MERCOSUR and the adaptation of regulations to integrate digital health innovations.
As the healthcare landscape in Latin America evolves, the regulatory environment for medical devices is undergoing significant transformations. With the region's medical device market projected to reach USD 37.23 billion by 2025, understanding the latest regulatory trends is crucial for industry stakeholders. This article delves into seven key trends shaping the future of medical device regulation in LATAM. It poses a critical question: how can manufacturers navigate these changes to ensure compliance while seizing new opportunities in a rapidly growing market?
bioaccess® spearheads the advancement of clinical research for healthcare instruments throughout Latin America. By leveraging the region's regulatory speed, bioaccess® secures ethical approvals in an impressive 4-6 weeks—an invaluable advantage for Medtech companies eager to capitalize on opportunities in LATAM's burgeoning healthcare sector. This swift approval process not only facilitates faster entry into the market but also directly supports the anticipated growth of the LATAM healthcare equipment industry, which is influenced by the medical device regulatory trends 2025 latam, projected to reach USD 37.23 billion by 2025.
Furthermore, bioaccess® is committed to conducting clinical trials with the utmost efficiency and adherence to quality standards, ensuring that innovative healthcare technologies can be developed and introduced without delay. With over 15 years of industry experience, bioaccess® not only streamlines the path to commercialization but also fosters innovation within the healthcare sector by providing tailored solutions that meet the unique needs of its clients.
Industry leaders emphasize that such regulatory flexibility is essential for addressing the rising demand for advanced healthcare technologies, particularly in a rapidly evolving market.
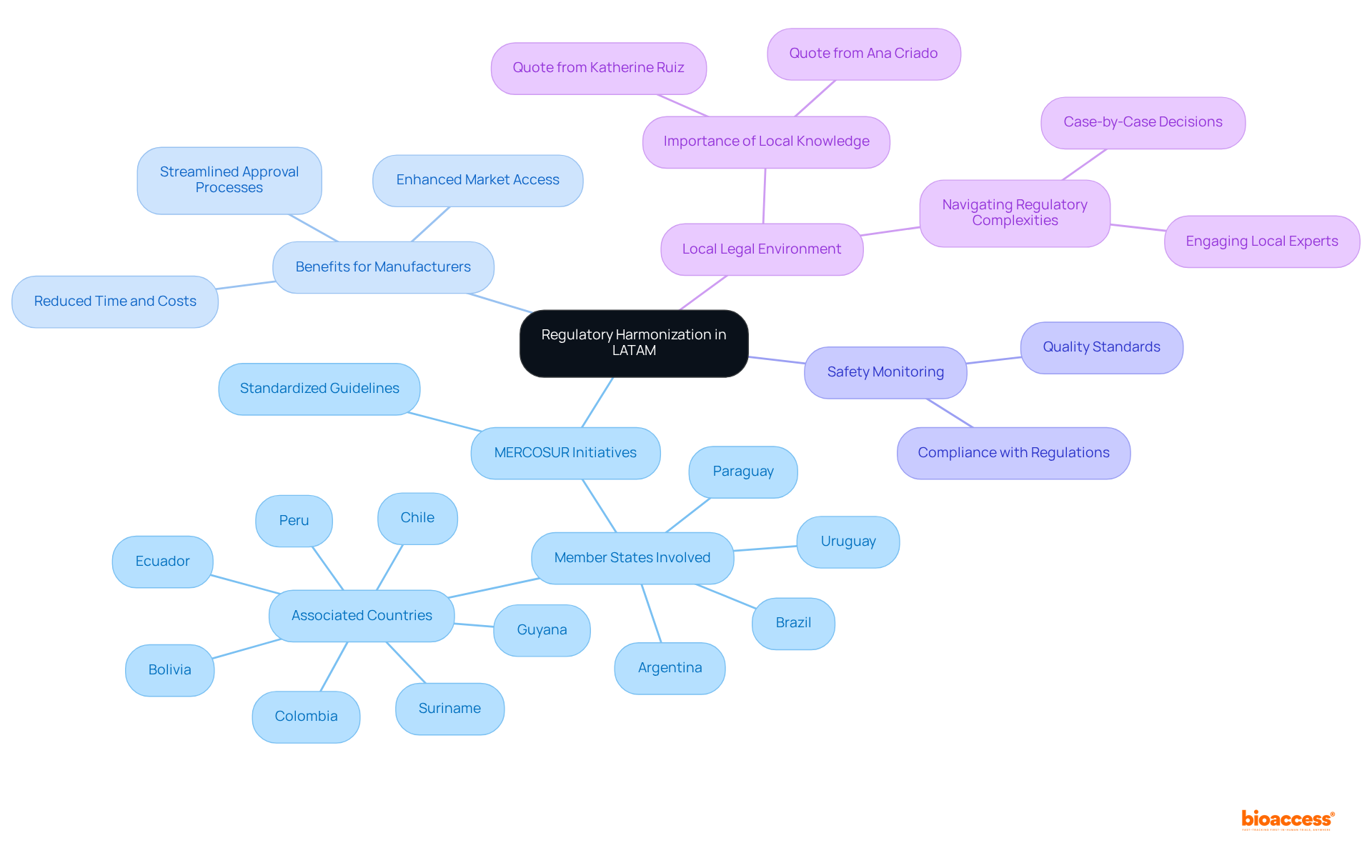
Regulatory harmonization stands as a pivotal trend in LATAM, reflecting the medical device regulatory trends 2025 latam aimed at streamlining the approval processes for healthcare equipment across multiple nations. Initiatives spearheaded by MERCOSUR, which includes Argentina, Brazil, Paraguay, and Uruguay, along with associated nations and PAHO, are fostering collaboration among member states. Their goal is to establish standardized guidelines in line with the medical device regulatory trends 2025 latam for the regulation of medical equipment. This unified effort not only simplifies the approval process but also fortifies the safety monitoring infrastructure, ensuring that devices meet consistent quality standards.
Consequently, manufacturers can navigate the compliance landscape shaped by medical device regulatory trends 2025 latam with enhanced efficiency, significantly reducing the time and costs associated with market entry. The impact of these initiatives is evident, as they enhance the overall governance framework, facilitating smoother interactions between innovators and regulatory bodies, ultimately accelerating access to essential medical technologies in the region.
As Katherine Ruiz, a Compliance Affairs specialist, notes, 'Navigating the local legal environment can be intricate, but possessing local knowledge can significantly enhance the speed of obtaining permissions.' Furthermore, with Latin America accounting for 3.9% of the global compliance affairs market in 2023, the significance of these harmonization efforts cannot be overstated.
As digital health technologies advance rapidly, the medical device regulatory trends 2025 LATAM are evolving to effectively accommodate these innovations. Regulations are being adapted to address the unique challenges posed by digital health solutions, including telemedicine and mobile health applications, in line with the medical device regulatory trends 2025 LATAM. This adaptation involves the creation of robust protocols for:
Consequently, manufacturers must remain vigilant regarding these policy changes to ensure compliance and fully leverage the benefits of digital health technologies in their offerings.
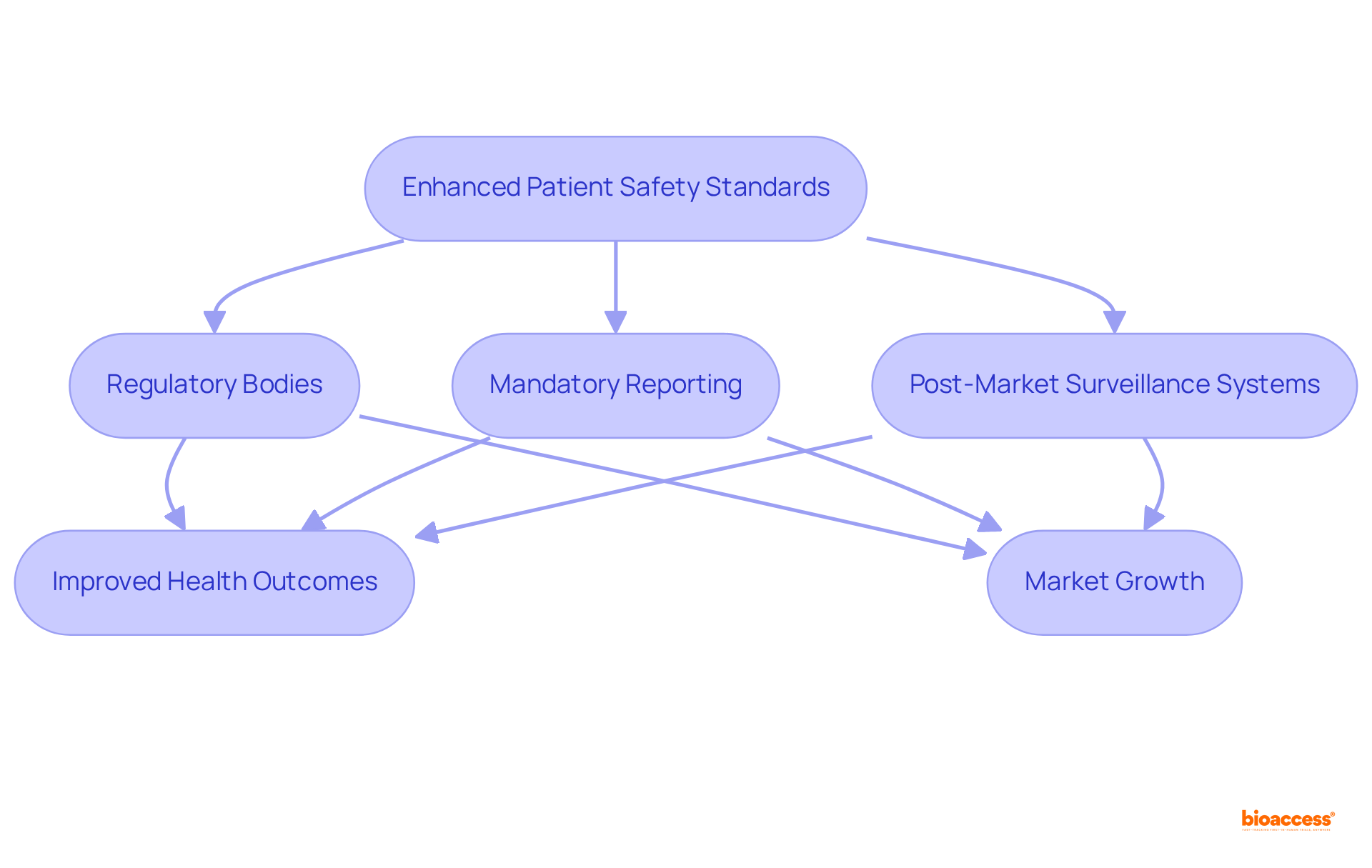
In LATAM, the enhancement of patient safety standards is increasingly vital within the regulatory framework, particularly in relation to the medical device regulatory trends 2025 latam, especially regarding the post-market monitoring of medical instruments.
Regulatory bodies are instituting more rigorous criteria for overseeing the safety and efficacy of products in line with medical device regulatory trends 2025 latam post-launch. This encompasses mandatory reporting of adverse events and the establishment of robust post-market surveillance systems.
Notably, the Latin America patient safety and risk management software market generated a revenue of USD 131.6 million in 2024 and is projected to grow at a compound annual growth rate (CAGR) of 12.6% from 2025 to 2030, reflecting the heightened emphasis on safety protocols.
By prioritizing patient safety, manufacturers not only ensure compliance with the medical device regulatory trends 2025 latam but also cultivate trust among healthcare providers and patients, ultimately leading to improved health outcomes.
Furthermore, the trend towards global harmonization of vigilance reporting standards, in light of medical device regulatory trends 2025 latam, simplifies compliance for multinational companies, underscoring the necessity for manufacturers to remain informed and adapt to these changes.
As artificial intelligence (AI) and machine learning technologies gain traction in healthcare tools, it is crucial to navigate the associated regulatory challenges. LATAM regulators are starting to formulate guidelines that address the unique aspects of AI, reflecting the medical device regulatory trends 2025 LATAM, including:
Manufacturers must adapt their compliance strategies to meet the medical device regulatory trends 2025 LATAM, ensuring that their AI-enabled products are safe, effective, and compliant with local regulations. This proactive approach is vital for maintaining a competitive edge in the swiftly advancing landscape of healthcare technology.
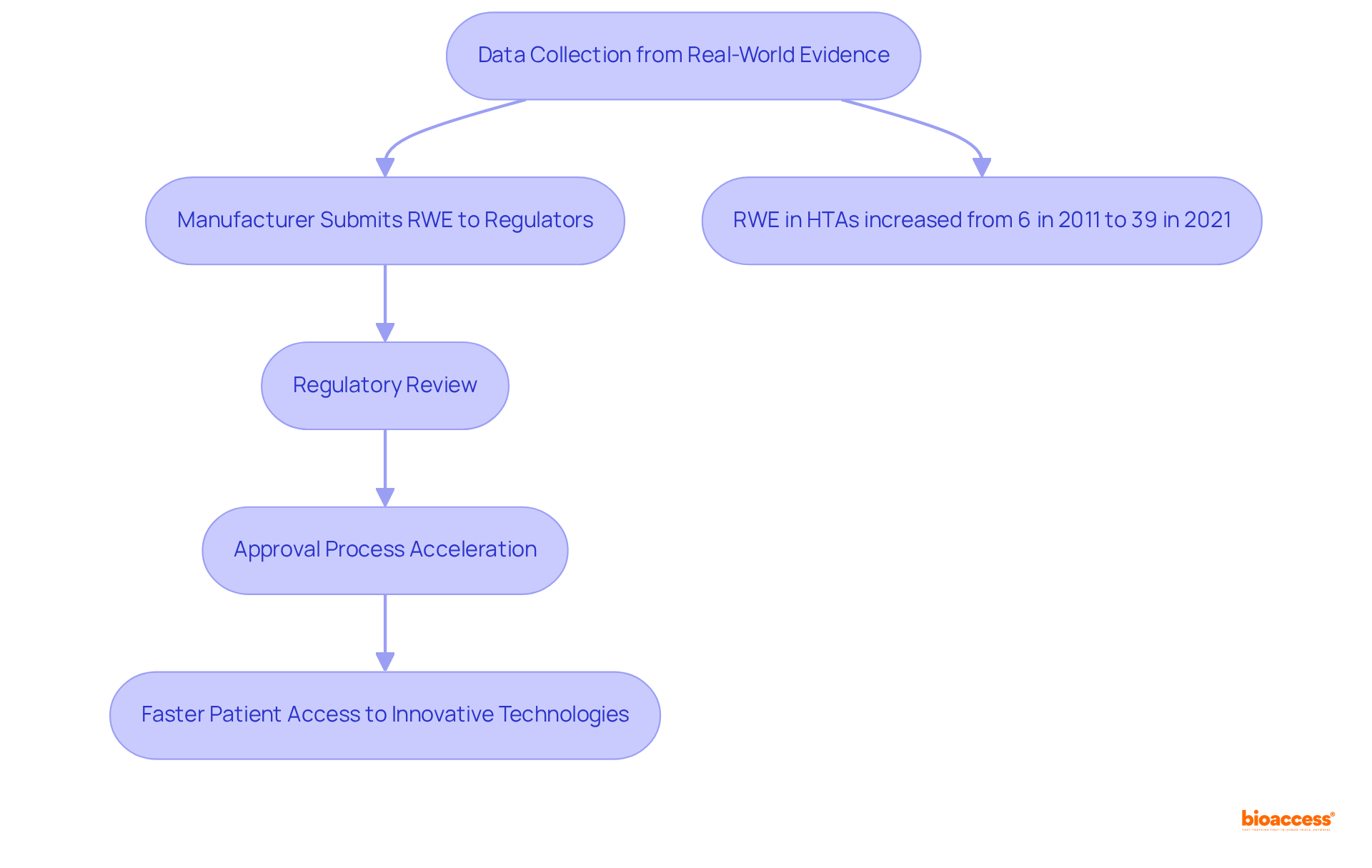
Real-world evidence (RWE) has emerged as an essential component in the approval process for medical equipment. By leveraging data collected from actual clinical environments, manufacturers can provide regulators with valuable insights into the safety and effectiveness of their products across diverse patient populations. This approach not only supplements traditional clinical trial data but also accelerates the approval process by demonstrating the practical benefits of devices in everyday use.
Notably, the application of RWE in Health Technology Assessments (HTAs) has increased from 6% in 2011 to 39% in 2021, signaling its growing acceptance in oversight processes. As oversight bodies in LATAM increasingly recognize the importance of RWE, manufacturers are urged to develop comprehensive strategies for gathering and analyzing this data to enhance their submissions in accordance with medical device regulatory trends 2025 latam.
For instance, the review of cemiplimab illustrated the drug's advantages through RWE, resulting in expedited approvals and improved market access. Nevertheless, challenges persist in the development of clinical evidence, particularly due to a limited number of patients available for recruitment and lengthy timelines for mature outcomes. This trend underscores the necessity of a proactive strategy in integrating RWE into official submissions, ultimately facilitating quicker patient access to innovative healthcare technologies.
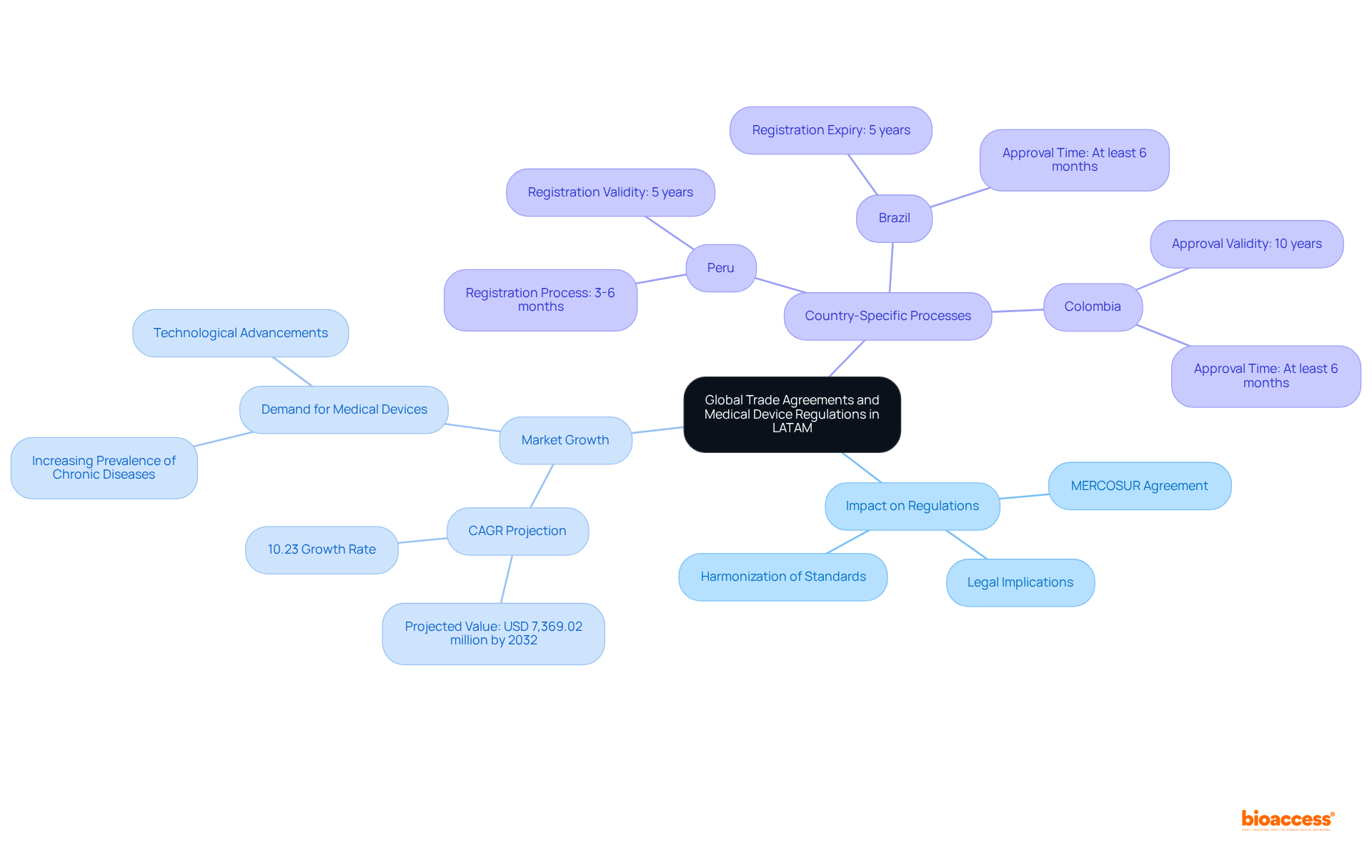
International trade agreements play a pivotal role in shaping the medical device regulatory trends 2025 LATAM, fostering regulatory consistency and collaboration among member nations. These agreements typically encompass provisions that enhance access for manufacturers by reducing tariffs and harmonizing standards. Notably, the Latin American Medical Equipment Contract Manufacturing Sector is projected to grow at a CAGR of 10.23% from 2024 to 2032, reaching a value of USD 7,369.02 million by 2032. This growth underscores the increasing competitiveness of the region for healthcare equipment firms.
As manufacturers navigate these agreements, vigilance regarding the legal implications and opportunities arising from international collaboration is essential. For instance, the MERCOSUR agreement seeks to standardize registration requirements across member countries, streamlining the approval process while ensuring compliance with quality standards. In Peru, registration processes span three to six months and expire after five years, whereas in Colombia, approvals typically require at least six months and are valid for ten years. This alignment in compliance not only facilitates easier entry into the industry but also enhances the overall efficiency of the medical device sector in LATAM, in accordance with the medical device regulatory trends 2025 latam.
In this dynamic landscape, companies must prioritize adherence to both local and international regulations to optimize their market potential while leveraging the benefits provided by these trade agreements. As Patricia M. Flood, a former compliance affairs specialist, remarked, "It is important for a company to be aware of the regulations and adhere to them, even if they are not yet enforced." Furthermore, the rising prevalence of chronic diseases in LATAM is propelling demand for medical devices, which highlights the critical nature of adhering to medical device regulatory trends 2025 latam in addressing healthcare needs.
The evolving landscape of medical device regulations in Latin America is characterized by significant trends that are fundamentally reshaping the industry as we approach 2025. These trends underscore the critical need for swift regulatory approvals and harmonization, while also highlighting the necessity for adaptability amidst rapid technological advancements. Companies that effectively navigate these shifts stand to gain immensely from the burgeoning opportunities within LATAM's healthcare sector.
Key insights from the article reveal the following:
These elements illustrate the growing complexity and imperative of compliance in this dynamic environment. Each of these elements is pivotal in enhancing the overall efficacy and safety of medical devices, ultimately benefiting both patients and healthcare providers.
As the medical device landscape continues to evolve, stakeholders must remain proactive and well-informed about regulatory trends and compliance challenges. Embracing these changes not only fosters innovation but also ensures that manufacturers can effectively meet the rising demand for advanced healthcare technologies in LATAM. By prioritizing adherence to regulatory standards and leveraging the advantages of emerging trends, companies can strategically position themselves for success in a rapidly transforming market.
What is bioaccess® and what role does it play in clinical research for medical devices in LATAM?
bioaccess® is an organization that accelerates clinical research for healthcare instruments throughout Latin America. It leverages the region's regulatory speed to secure ethical approvals in 4-6 weeks, benefiting Medtech companies looking to enter LATAM's healthcare sector.
How does bioaccess® impact the entry of medical devices into the LATAM market?
The swift approval process facilitated by bioaccess® allows for faster market entry of medical devices, supporting the growth of the LATAM healthcare equipment industry, which is projected to reach USD 37.23 billion by 2025.
What is the commitment of bioaccess® regarding clinical trials?
bioaccess® is committed to conducting clinical trials efficiently and adhering to quality standards, ensuring that innovative healthcare technologies can be developed and introduced without delay.
How does regulatory harmonization benefit medical device approvals in LATAM?
Regulatory harmonization aims to streamline the approval processes for healthcare equipment across multiple nations in LATAM, simplifying compliance and enhancing safety monitoring, which ultimately accelerates access to medical technologies.
What initiatives are contributing to regulatory harmonization in LATAM?
Initiatives spearheaded by MERCOSUR, which includes Argentina, Brazil, Paraguay, and Uruguay, along with associated nations and PAHO, are fostering collaboration to establish standardized guidelines for regulating medical equipment.
What are the advantages of regulatory harmonization for manufacturers?
Regulatory harmonization enhances efficiency in navigating compliance landscapes, significantly reducing the time and costs associated with market entry for manufacturers of medical devices.
What is the significance of local knowledge in obtaining permissions within LATAM?
Local knowledge is crucial for navigating the intricate local legal environment, significantly enhancing the speed of obtaining permissions for medical device approvals, as noted by industry experts.
What is the current market share of Latin America in the global compliance affairs market?
In 2023, Latin America accounted for 3.9% of the global compliance affairs market, highlighting the importance of regulatory harmonization efforts in the region.