


The article examines various software solutions tailored to streamline clinical trials, effectively addressing challenges such as regulatory compliance, participant recruitment, and data management. It emphasizes specific systems like bioaccess® and InfoEd, which significantly enhance efficiency through rapid ethical approvals and comprehensive management tools. This ultimately leads to improved trial outcomes and expedited patient enrollment, underscoring the critical role these technologies play in the Medtech landscape. By leveraging such solutions, clinical researchers can navigate complexities more effectively, fostering collaboration and driving innovation in trial processes.
The rapid evolution of clinical trials is reshaping the landscape of medical research, with technology playing a pivotal role in enhancing efficiency and effectiveness. As the demand for innovative therapies grows, the integration of software solutions has become essential for streamlining processes and overcoming common challenges faced during trials. However, with numerous options available, how can organizations identify the most effective tools to optimize their research efforts and ensure successful outcomes? This article explores seven cutting-edge software solutions that promise to revolutionize clinical trials, offering insights into their unique benefits and the transformative impact they can have on the research process.
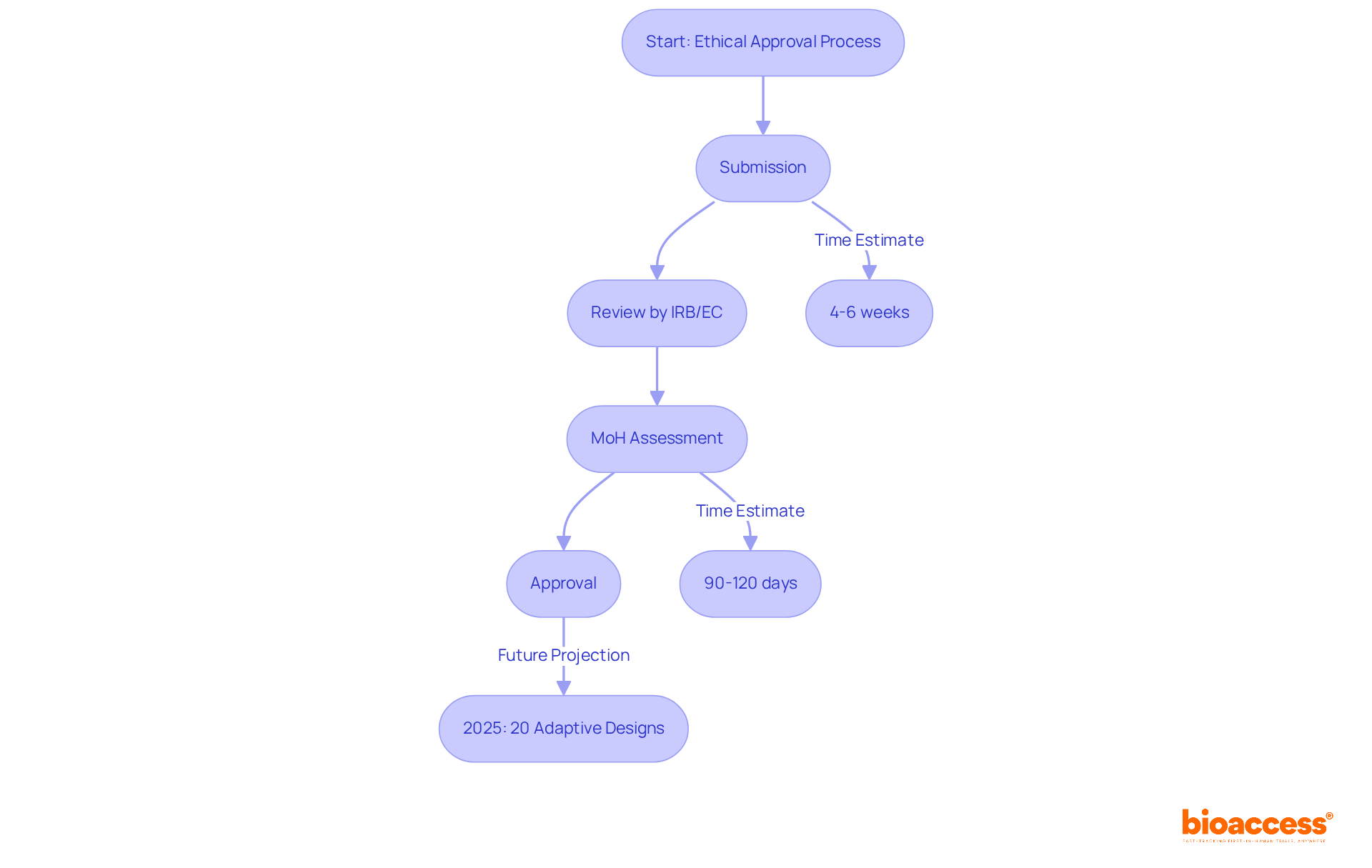
bioaccess® distinguishes itself in the research sector by delivering swift ethical approvals, typically within 4-6 weeks. This impressive speed is made possible by the regulatory frameworks in Latin America, especially in Colombia, which are designed to accelerate approvals while upholding stringent ethical standards. The overall IRB/EC and MoH (INVIMA) assessment in Colombia spans 90-120 days, enabling bioaccess® to assist Medtech, Biopharma, and Radiopharma innovators in launching their trials more promptly. This capability significantly enhances the pathway to commercialization and improves access to innovative therapies through software clinical trials.
Looking ahead to 2025, it is projected that approximately 20% of software clinical trials in Latin America will adopt adaptive designs, a trend that bioaccess® is ideally positioned to support through its agile regulatory strategies. As Julio Martinez-Clark, CEO of bioaccess®, aptly notes, "Medtech companies are increasingly seeking expedited regulatory approval, ease of participant recruitment, and cost efficiency." With a population exceeding 50 million and 95% coverage under universal healthcare, Colombia presents a robust landscape for patient recruitment.
Moreover, Colombia's healthcare system ranks #22 according to the World Health Organization, and hospitals are required to undergo a rigorous ICH/GCP certification process to conduct research. Additionally, investments in science, technology, and innovation initiatives in Colombia benefit from substantial R&D tax incentives, including a 100% tax deduction and various credits. This renders the country an attractive location for medical studies. Such strategic emphasis not only accelerates testing timelines but also contributes to achieving higher success rates in introducing new therapies to the market. Furthermore, the medical information interoperability market is expected to grow at a 12.9% CAGR, underscoring the increasing importance of effective research procedures in the region.
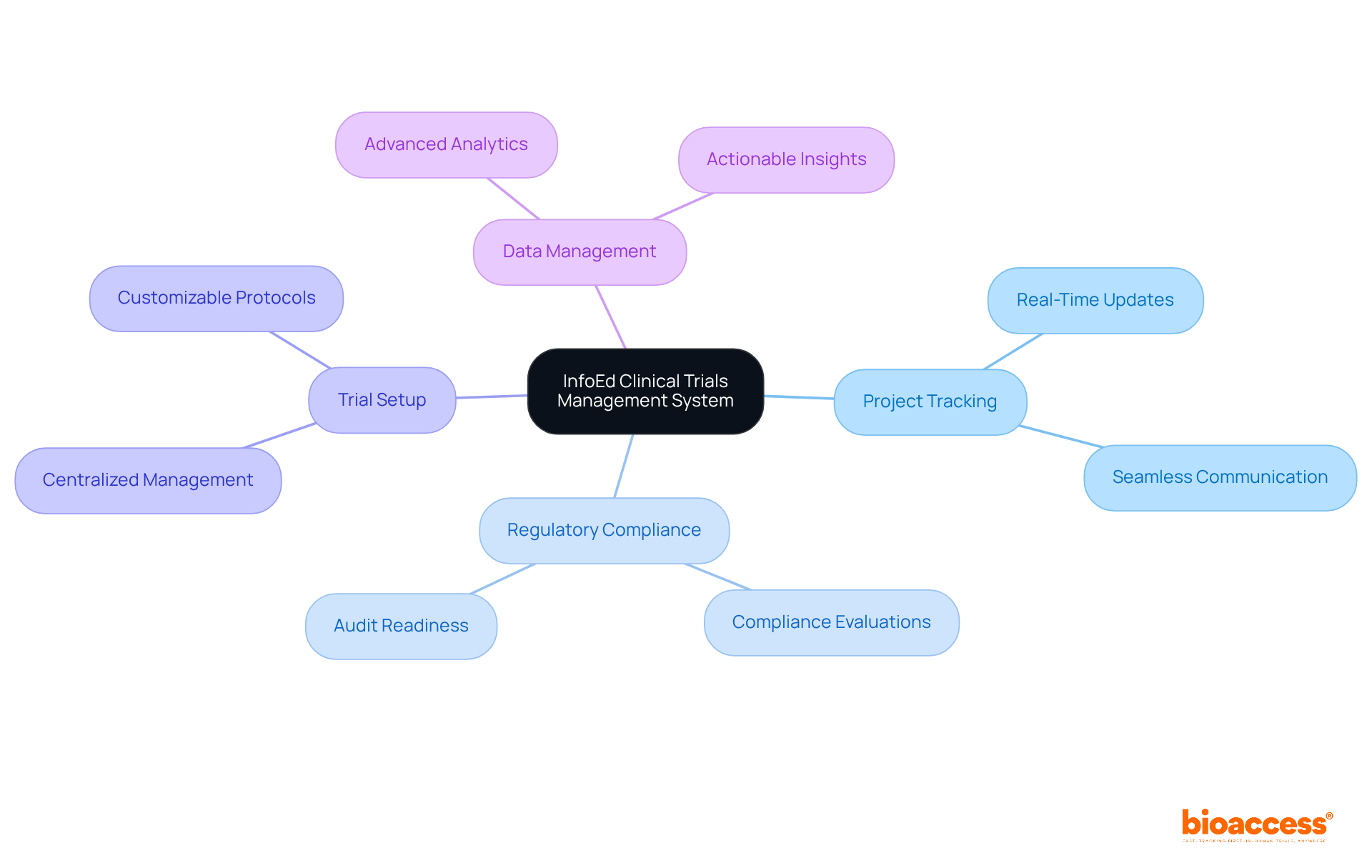
The InfoEd Clinical Studies Management System offers a robust suite of tools designed to significantly enhance the management of software clinical trials. This software clinical trials solution effectively streamlines project tracking, regulatory compliance, trial setup, and data management, ensuring that every aspect of the trial is organized and easily accessible. By integrating multiple functionalities into a single platform, InfoEd empowers project teams to maintain oversight and boost efficiency in software clinical trials, ultimately leading to superior outcomes and a reduction in administrative burdens.
For instance, medical study directors have noted that the centralized nature of InfoEd facilitates real-time updates and seamless communication among team members, which is essential for prompt decision-making. Additionally, recent updates to the InfoEd system have introduced advanced analytics features, enabling teams to extract actionable insights from research data more effectively. This thorough approach not only enhances the management of experiments but also elevates the overall standard of medical investigation.
Moreover, bioaccess's capabilities in feasibility assessments, site selection, and compliance evaluations expedite software clinical trials, resulting in 50% quicker patient enrollment and $25K savings with FDA-ready data. A research director remarked, "The incorporation of InfoEd has transformed our study management processes, allowing us to focus more on research and less on administrative tasks." This underscores the system's impact on operational efficiency.
To maximize the benefits of InfoEd's features, teams should consistently review the analytics dashboard to identify trends and make informed decisions throughout the process.
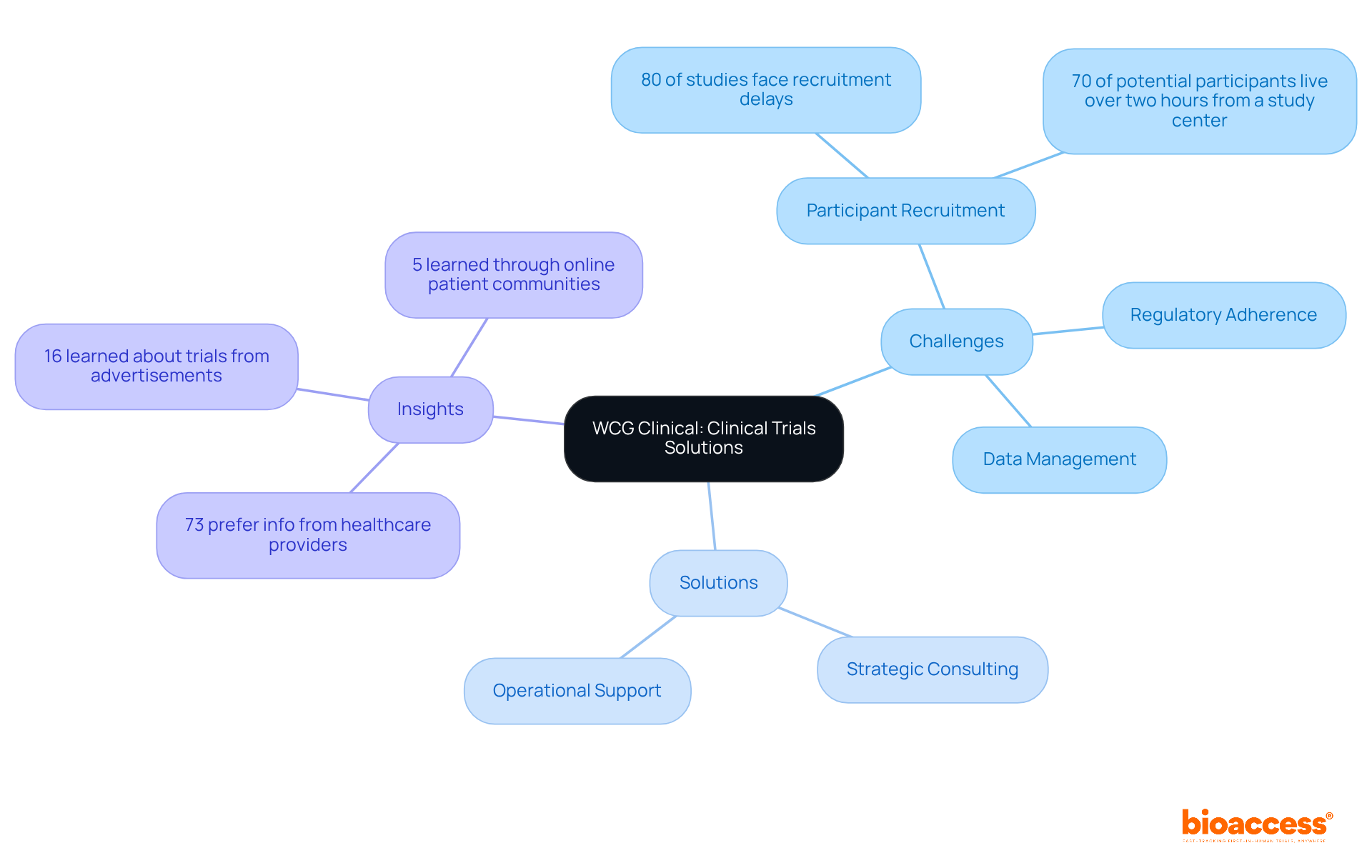
WCG Clinical excels in addressing common obstacles encountered during research studies, particularly in participant recruitment, regulatory adherence, and data management. Their tailored solutions, which include strategic consulting and operational support, enable teams to effectively navigate these challenges. Notably, approximately 80% of clinical studies experience delays or terminations due to recruitment difficulties, making WCG Clinical's expertise essential.
By employing innovative strategies, they empower organizations to enhance efficiency in testing and achieve their research objectives. Organizations utilizing WCG Clinical solutions have reported significantly improved success rates in participant enrollment, underscoring the effectiveness of their customized approaches.
Furthermore, WCG Clinical emphasizes the importance of engaging patients through various channels, as 73% of participants prefer to receive information about studies from their healthcare providers. This insight highlights the necessity for effective communication strategies in recruitment efforts.
Overall, WCG Clinical's commitment to refining research processes positions them as a vital partner in advancing medical studies.
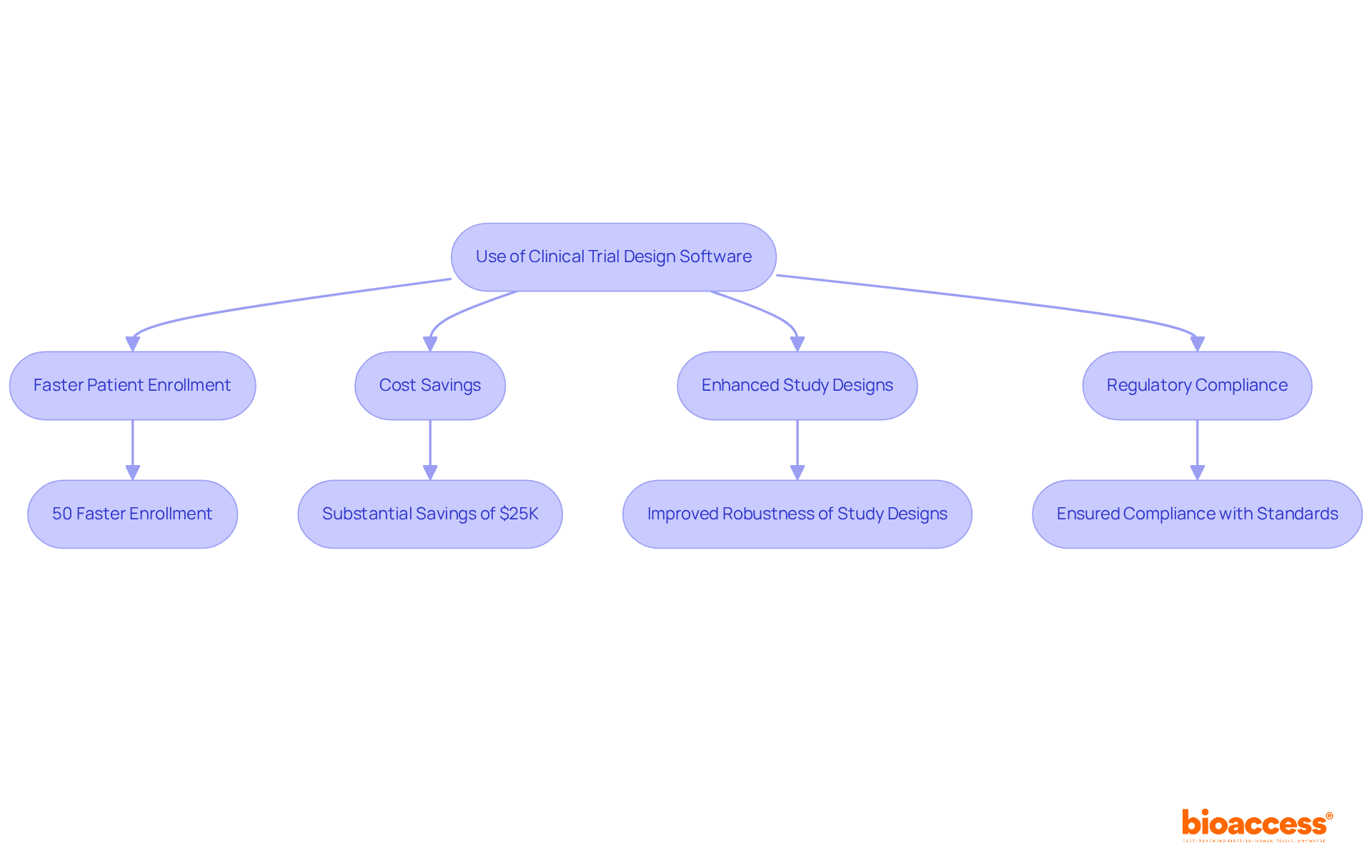
Software clinical trials are essential for optimizing study protocols in clinical research. These sophisticated tools empower researchers to simulate diverse scenarios, evaluate potential outcomes, and refine methodologies prior to implementation. By leveraging software clinical trials, research teams can significantly enhance the robustness of their study designs, ensuring compliance with regulatory standards and producing meaningful results that advance medical knowledge.
Notably, with bioaccess®, medical studies can achieve patient enrollment 50% faster than traditional Western sites, translating into substantial cost savings of $25K per patient through FDA-ready data—eliminating rework and delays. As Stefan Harrer aptly stated, "AI has the potential to transform essential phases of study design from preparation to execution towards enhancing success rates." This insight is particularly critical given that "only one of 10 compounds entering a medical study reaches the market," underscoring the necessity of refined study designs in mitigating high failure rates.
Furthermore, the financial implications are staggering, with losses per unsuccessful research project ranging from 800 million to 1.4 billion USD. By utilizing software clinical trials, organizations not only boost their operational efficiency but also foster a more patient-centric approach in investigations, ultimately facilitating the development of more effective therapies that reach the market.
Recruitment and monitoring tools are essential for enhancing participant involvement throughout the research study process. These tools facilitate targeted outreach, streamline communication, and effectively monitor participant progress. By implementing advanced recruitment strategies—such as leveraging social media platforms with over 3 billion active users—research teams can significantly boost enrollment rates.
For instance, research shows that 73% of patients prefer to learn about clinical studies through their doctor's office, underscoring the importance of direct communication in recruitment efforts. Moreover, tracking systems that provide real-time updates keep participants informed and engaged, ultimately leading to more successful study outcomes.
Clinical study coordinators assert that effective recruitment strategies not only attract participants but also foster a sense of trust and commitment, which is crucial for sustaining engagement throughout the research.
Efficient oversight of research project budgets is crucial in clinical research, relying heavily on sophisticated accounting management tools. These tools meticulously track expenses, accurately forecast costs, and ensure compliance with financial regulations. By leveraging robust financial management systems alongside comprehensive study management services from bioaccess®, which include feasibility assessments, site selection, and compliance evaluations, organizations can maintain stringent control over their budgets. This approach significantly minimizes financial risks and enhances resource distribution.
The strategic integration of these tools not only facilitates the successful execution of software clinical trials but also enhances overall operational efficiency. Financial managers in healthcare emphasize the necessity of real-time budget tracking tools, which enable prompt adjustments and informed decision-making. As Vedant Srivastava noted, '91% of study sites indicate that delayed sponsor or CRO payments create operational challenges,' underscoring the urgency of addressing financial management issues.
Furthermore, with patient recruitment accounting for approximately $1.89 billion of the total clinical trial budget, the adoption of innovative financial management solutions, in conjunction with bioaccess®'s tailored services, is increasingly vital for achieving success in software clinical trials and ensuring timely patient enrollment.
A Clinical Trials Document Management System (DMS) is essential for ensuring compliance and organization throughout the study process. These systems enable the secure storage, retrieval, and sharing of vital documents, including protocols, consent forms, and regulatory submissions. By adopting a robust DMS, research teams can streamline workflows, ensuring adherence to regulatory standards while enhancing collaboration among stakeholders.
As the research study environment evolves, particularly leading up to 2025, the incorporation of sophisticated document management solutions becomes increasingly vital. These systems not only facilitate compliance but also improve operational efficiency by automating document tracking and version control.
Organizations like bioaccess, a leading Contract Research Organization in Latin America, have implemented comprehensive DMS solutions that report significant reductions in compliance violations and enhanced audit readiness. By centralizing documentation and offering immediate access to essential information, a DMS fosters a culture of openness and responsibility, which is crucial for successful medical studies.
Furthermore, bioaccess's extensive capabilities in feasibility studies, site selection, compliance reviews, setup, import permits, project management, and reporting ensure that studies are conducted efficiently and effectively.
Cooperation between investigators and coordinators is essential for the success of clinical studies. Investigators uphold the scientific integrity of the study, while coordinators manage daily operations such as participant recruitment and data collection. This collaboration is crucial for carrying out tests effectively and ethically, resulting in dependable outcomes and enhanced patient benefits.
Effective communication and common objectives between these roles promote a unified atmosphere that improves performance. For example, when coordinators engage with investigators during protocol development, they can identify potential challenges early, streamlining recruitment and ensuring adherence to regulatory standards.
Katherine, an expert in regulatory affairs at bioaccess®, illustrates this synergy; her experience in securing market approval for medical innovations highlights the essential role of regulatory adherence in research studies. As Christina Brennan pointed out, 'It doesn’t take a rocket scientist to see the value of certification,' underscoring the significance of continuous training and teamwork in the research environment.
This synergy not only enhances operational efficiency but also aids in higher participant retention rates and data integrity, emphasizing the importance of collaboration in attaining research success. Furthermore, bioaccess® provides extensive services such as:
These services further improve the efficiency of research activities.
To enhance efficiency in software clinical trials, it is crucial to alleviate administrative burdens by streamlining processes and optimizing workflows. This objective can be realized through the implementation of:
Notably, with bioaccess®, teams can enroll treatment-naive cardiology or neurology cohorts 50% quicker than their Western counterparts, resulting in $25K savings per patient with FDA-ready data—no rework, no delays. By minimizing these administrative challenges, research teams can focus on essential tasks in software clinical trials, which ultimately leads to faster study completion and improved data quality.
The landscape of clinical study management has undergone a significant transformation, with contemporary approaches to software clinical trials emphasizing flexibility, technology integration, and patient-centric strategies. Traditional methods often relied on manual processes and rigid protocols, which could hinder efficiency and responsiveness.
For instance, organizations that have adopted AI-driven tools have reported substantial reductions in participant recruitment timelines; one AI matching system successfully linked 16 cardiac study participants in just one hour. This transition not only fosters collaboration but also improves overall outcomes, ensuring that organizations remain competitive in a swiftly evolving environment.
With the clinical research market projected to reach approximately USD 886.5 billion by 2032, the demand for adaptive strategies in software clinical trials has become increasingly critical. Clinical research directors assert that embracing these modern practices is vital for navigating the complexities of today's software clinical trials, ultimately leading to more effective and timely medical advancements.
The integration of innovative software solutions in clinical trials is transforming the research landscape, offering unprecedented efficiency and effectiveness in study management. By leveraging tools that streamline processes, enhance participant engagement, and optimize resource allocation, organizations can significantly improve their outcomes and accelerate the path to market for new therapies.
Key insights from the article highlight the importance of various software solutions, such as:
Each of these tools addresses specific pain points in clinical research, from budget management to regulatory compliance, ultimately fostering a more agile and responsive research environment.
As the clinical trials sector continues to evolve, embracing modern management practices and adaptive strategies becomes crucial. Organizations must prioritize the adoption of advanced technologies and effective communication methods to navigate the complexities of today’s research landscape. By doing so, they not only enhance their operational efficiency but also contribute to the advancement of medical science, ensuring that innovative therapies reach patients more swiftly and effectively.
What is bioaccess® and what services does it provide?
bioaccess® specializes in accelerating clinical trials by delivering rapid ethical approvals, typically within 4-6 weeks, particularly in Latin America, especially Colombia. They assist Medtech, Biopharma, and Radiopharma innovators in launching their trials more promptly.
How does bioaccess® achieve quick ethical approvals?
The quick ethical approvals are made possible by the regulatory frameworks in Latin America, specifically Colombia, which are designed to expedite approvals while maintaining stringent ethical standards.
What is the typical timeline for IRB/EC and MoH (INVIMA) assessment in Colombia?
The overall assessment in Colombia typically spans 90-120 days.
What is the projected trend for software clinical trials in Latin America by 2025?
It is projected that approximately 20% of software clinical trials in Latin America will adopt adaptive designs by 2025.
What advantages does Colombia offer for clinical trials?
Colombia has a population exceeding 50 million with 95% universal healthcare coverage, making it an attractive location for patient recruitment. Additionally, the country's healthcare system is well-regarded, and hospitals must undergo rigorous ICH/GCP certification to conduct research.
What are the benefits of R&D tax incentives in Colombia?
Investments in science, technology, and innovation initiatives in Colombia benefit from substantial R&D tax incentives, including a 100% tax deduction and various credits, making it a favorable environment for medical studies.
How does the InfoEd Clinical Studies Management System enhance clinical trial management?
The InfoEd system streamlines project tracking, regulatory compliance, trial setup, and data management, integrating multiple functionalities into a single platform to enhance oversight and efficiency in software clinical trials.
What features have been introduced in recent updates to the InfoEd system?
Recent updates have introduced advanced analytics features, enabling teams to extract actionable insights from research data more effectively.
How does bioaccess® contribute to faster patient enrollment in clinical trials?
bioaccess®'s capabilities in feasibility assessments, site selection, and compliance evaluations expedite software clinical trials, resulting in 50% quicker patient enrollment and $25K savings with FDA-ready data.
What challenges does WCG Clinical address in clinical trials?
WCG Clinical addresses common obstacles in participant recruitment, regulatory adherence, and data management, providing tailored solutions that enhance efficiency and success rates in testing.
Why is participant recruitment a significant challenge in clinical studies?
Approximately 80% of clinical studies experience delays or terminations due to recruitment difficulties, highlighting the need for effective strategies in this area.
What communication strategies does WCG Clinical emphasize for patient engagement?
WCG Clinical emphasizes engaging patients through various channels, noting that 73% of participants prefer to receive information about studies from their healthcare providers.