


The article emphasizes strategies to advance research in rare and orphan diseases, clearly stating its purpose and relevance to clinical research. It highlights the critical importance of collaboration, regulatory navigation, and innovative approaches in clinical trials.
By leveraging strategic partnerships and streamlined regulatory processes, the article asserts that the speed and effectiveness of developing new therapies can be significantly enhanced. This ultimately leads to improved patient outcomes and addresses the urgent needs of those affected by these conditions.
The insights provided underscore the necessity for a united effort in tackling the challenges faced in the Medtech landscape, reinforcing the call for action in this vital area of research.
The landscape of rare disease and orphan disease research is evolving rapidly, driven by the urgent need for innovative therapies and effective treatment strategies. With approximately 300 million individuals affected globally, the stakes are high, and the challenges are multifaceted, ranging from regulatory hurdles to funding limitations. This article explores seven key strategies that can significantly advance research efforts in this critical area, raising questions about how collaborative approaches and technological advancements can reshape the future of care for those impacted by these conditions.
bioaccess® excels in accelerating clinical studies for rare disease and orphan disease by leveraging its strategic sites across Latin America, the Balkans, and Australia. With ethical approvals secured in an impressive 4-6 weeks and enrollment processes that are 50% faster than traditional markets, bioaccess® provides a distinct advantage for innovators in the Medtech, Biopharma, and Radiopharma sectors. This rapid approach not only expedites the delivery of treatments to the market but also addresses the urgent needs of individuals affected by rare disease and orphan disease, ensuring that innovative therapies are accessible sooner than ever.
The recent success of the SonoThrombectomy™ system in Chile exemplifies how swift clinical trials can lead to significant advancements in treatment options for venous thromboembolism, underscoring the potential of Latin America as a hub for innovative studies. Additionally, strategic initiatives by organizations like COFEPRIS, which has reduced review times for Phase III clinical trials to just 45 days, further enhance the region's attractiveness for conducting urgent clinical studies.
Through its commitment to high standards and rapid execution, bioaccess® is at the forefront of transforming the landscape of studies on rare disease and orphan disease, making a profound impact on the lives of patients worldwide.
Orphan conditions, classified as rare disease and orphan disease affecting fewer than 200,000 individuals in the U.S., pose significant challenges in healthcare due to their rarity and the limited funding available for research. Approximately 300 million people worldwide are affected by these conditions, which often lead to severe health complications and necessitate specialized treatment approaches. Notably, the average time for an individual with a rare condition to receive a diagnosis is around five years, underscoring the urgent need for enhanced research initiatives.
Advancing studies in this domain is essential, as it directly impacts the quality of life for those affected and their families. Despite the hurdles, such as high development costs and limited financial returns, the pharmaceutical industry is increasingly channeling investments into the development of drugs for rare disease and orphan disease. Projections indicate that by 2025, funding for research on rare disease and orphan disease will rise, driven by legislative support like the Orphan Drug Act, which has facilitated the approval of over 880 orphan medications since its inception.
Healthcare professionals emphasize the importance of understanding rare disease and orphan disease conditions, noting that many stem from single gene mutations and often present complex symptoms. The collaboration among government entities, pharmaceutical companies, and advocacy groups is vital for overcoming the challenges related to research and treatment. As advancements in genetic therapies and sophisticated diagnostic techniques continue to emerge, the potential for improving health outcomes for patients with rare conditions becomes increasingly promising.
Navigating the regulatory environment for uncommon condition research presents significant challenges due to stringent requirements and diverse regulations across regions. Organizations must adhere to both local and international regulations while addressing the unique features of rare disease and orphan disease. In Brazil, for example, the execution of the RDC 205 pathway has significantly decreased the average approval time for uncommon condition therapies to 246 days, almost half of the earlier duration. This expedited process not only enhances access to innovative treatments but also allows manufacturers to define pricing strategies earlier in the global launch sequence.
bioaccess® stands out in this complex environment by leveraging its extensive knowledge of regulatory processes in Latin America. The organization excels in delivering ethical approvals in just 4-6 weeks and facilitating enrollment that is 50% faster than traditional markets. By scheduling initial meetings with ANVISA within 60 days of submission to other regulatory agencies, bioaccess® ensures that clients can navigate the regulatory maze efficiently, aligning product information and submission documents effectively.
The 505(b)(2) pathway in the U.S. further exemplifies a streamlined approach, accounting for 40% of all new FDA approvals in 2024. This pathway allows developers to leverage existing data while introducing meaningful improvements, significantly reducing development costs and timelines. Such regulatory frameworks are crucial for promoting uncommon condition studies, as they offer a structured yet adaptable route for innovative therapies.
In summary, understanding and navigating the regulatory challenges associated with studies of drugs for rare disease and orphan disease is crucial for researchers aiming to bring new therapies to market. With its customized strategy and profound regulatory understanding, bioaccess® enables innovators to navigate these obstacles and speed up the creation of transformative therapies for rare disease and orphan disease.
Key Services Offered by bioaccess®:
By utilizing these services, researchers can improve their likelihood of success in the complicated field of uncommon condition research.
European Reference Networks (ERNs) are pivotal in advancing treatment for individuals with rare disease and orphan disease, fostering collaboration among healthcare professionals, researchers, and individuals throughout Europe. These networks are specifically designed to improve diagnosis, treatment, and access to specialized care, guaranteeing that individuals receive the optimal support they need. By facilitating knowledge exchange and collaborative research, ERNs bridge gaps in treatment and investigation, leading to improved health outcomes for those affected by uncommon conditions.
With an estimated 6,000 to 8,000 distinct conditions classified as rare diseases and orphan diseases impacting millions—around 80% of which are of genetic origin and 70% manifesting in childhood—the establishment of ERNs is essential for coordinating efforts across countries and regions. This coordination ultimately enhances the quality of care and individual experiences.
Healthcare providers assert that integrating ERNs into national healthcare systems not only streamlines access to expertise but also significantly boosts patient outcomes. Patients who feel respected and supported are more likely to adhere to treatment plans. As Theodore Roosevelt noted, the empathy and awareness provided by healthcare workers are crucial, underscoring the importance of a collaborative approach in addressing the complexities of rare disease and orphan disease, and ensuring that innovative treatments reach those in need.
Furthermore, the EU's investment of over €3.2 billion in research on rare disease and orphan disease from 2007 to 2020 underscores the commitment to improving care through these networks.
Recognizing unfulfilled needs in rare disease and orphan disease is essential for directing research efforts and ensuring that new treatments effectively address the most critical obstacles faced by individuals. Engaging with individuals receiving care, healthcare professionals, and researchers is crucial for gathering insights on current treatment gaps and areas that require innovation.
For instance, a recent study underscored that over 300 million individuals worldwide suffer from rare disease and orphan disease, emphasizing the urgency of addressing these unmet needs. By prioritizing these insights, organizations like bioaccess® can leverage their expertise in accelerated clinical trials to focus their efforts on developing therapies that not only meet regulatory requirements but also significantly enhance outcomes for individuals.
bioaccess® offers comprehensive clinical trial management services, including:
These services are vital for navigating the complexities of uncommon condition research in Latin America, Eastern Europe, and Australia. Furthermore, initiatives like the FDA's Accelerating Rare Disease and Orphan Disease Cures (ARC) aim to streamline the clinical trial process for rare disease and orphan disease therapies, ensuring that groundbreaking treatments reach individuals more efficiently. This collaborative approach fosters a deeper understanding of the intricacies related to uncommon conditions and propels the development of targeted solutions that can transform healthcare.
Research and Development (R&D) is fundamental to the creation of effective therapies for rare disease and orphan disease. This process necessitates extensive preclinical and clinical studies to assess safety and efficacy. The distinct obstacles presented by rare disease and orphan disease require research and development approaches specifically designed to address the needs of small groups of individuals. Notably, orphan drugs benefit from a higher approval rate of approximately 25%-30%, thanks to incentives from regulatory bodies like the FDA. However, the development process remains complex, with only about 10% of drugs entering clinical trials ultimately receiving FDA approval.
Organizations such as bioaccess® play a pivotal role in this landscape by providing comprehensive clinical trial management services. Their offerings include:
Their ability to deliver ethical approvals in just 4-6 weeks and facilitate enrollment that is 50% faster than traditional markets ensures that promising therapies can advance through the development pipeline efficiently. Engaging with industry leaders underscores the importance of addressing these challenges; as one expert noted, "the need for innovative study designs and patient-centric approaches is crucial for overcoming the hurdles in developing orphan drugs."
In summary, collaboration and a focus on innovative strategies are essential for navigating the complexities of drug development for rare disease and orphan disease. As the industry evolves, it is imperative for stakeholders to consider these insights and take action to enhance the effectiveness of clinical research.
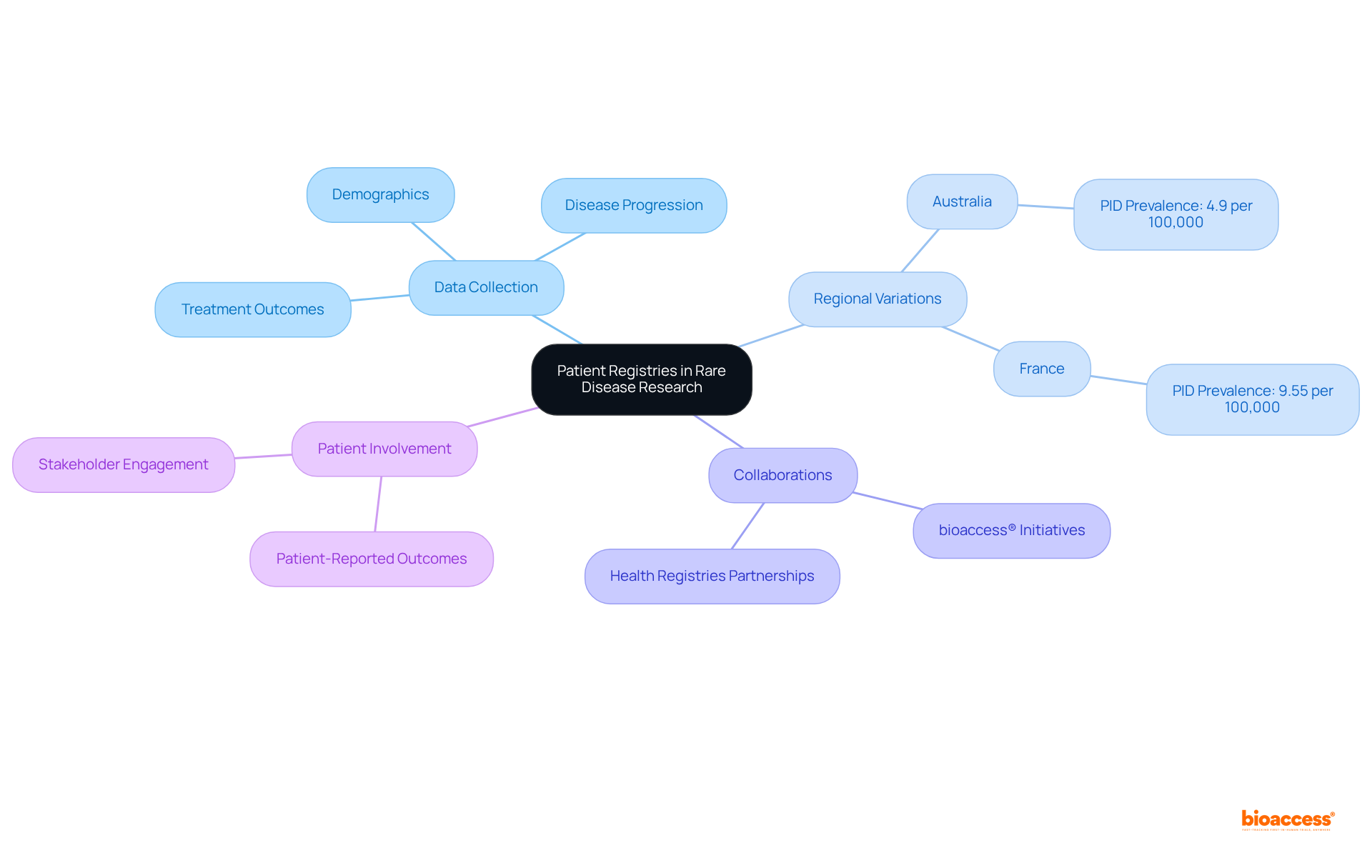
Registry systems play a pivotal role in advancing research on rare disease and orphan disease by systematically collecting and preserving comprehensive data on demographics, disease progression, and treatment outcomes. These registries empower researchers to identify trends, evaluate treatment effectiveness, and develop targeted therapies tailored to individual needs. For instance, the prevalence of primary immunodeficiencies (PID) exhibits significant regional variation, with estimates of 4.9 per 100,000 in Australia compared to 9.55 per 100,000 in France. This underscores the critical importance of localized data in understanding the impact of diseases.
By collaborating with health registries, organizations such as bioaccess® can enhance their initiatives, ensuring that studies are grounded in real-world data and the lived experiences of individuals. This collaboration not only accelerates the development of innovative therapeutic options but also improves treatment outcomes for patients with rare disease and orphan disease. Experts in the field emphasize that integrating patient-reported outcomes is essential for steering research and therapeutic strategies, ultimately leading to more effective interventions. Continuous evaluation and adaptation of these registries are crucial for maintaining their relevance and efficacy in the evolving landscape of research on rare disease and orphan disease.
Cooperation within the uncommon illness community is vital for fostering innovation and enhancing study initiatives. By uniting researchers, healthcare professionals, advocacy organizations, and industry participants, cooperative initiatives can leverage diverse expertise and resources to address the unique challenges associated with rare disease and orphan disease. The Rare Disease Research Partnership (RAinDRoP) exemplifies how inclusive discussions among various stakeholders can lead to the identification of critical priorities, such as support at the time of diagnosis and the need for effective diagnostic tests.
Organizations like bioaccess® play a crucial role in facilitating these collaborations, ensuring that all parties align their efforts toward shared objectives. This synergy accelerates the development of new therapies while improving care by incorporating insights from those directly affected by both rare disease and orphan disease. The emphasis on co-designing research and services with individuals and families underscores the importance of a user-centered approach, which is essential for meaningful advancements in treatment options.
Furthermore, the impact of partnerships on innovation is evident in the progress made in gene therapies and the increasing focus on data sharing. Industry leaders emphasize that promoting collaboration among researchers, clinicians, and manufacturers is crucial for enhancing access to treatments and ensuring that innovations yield tangible benefits for individuals. By prioritizing teamwork, the community for rare disease and orphan disease can continue to make significant strides in addressing the unmet needs of those living with these conditions.
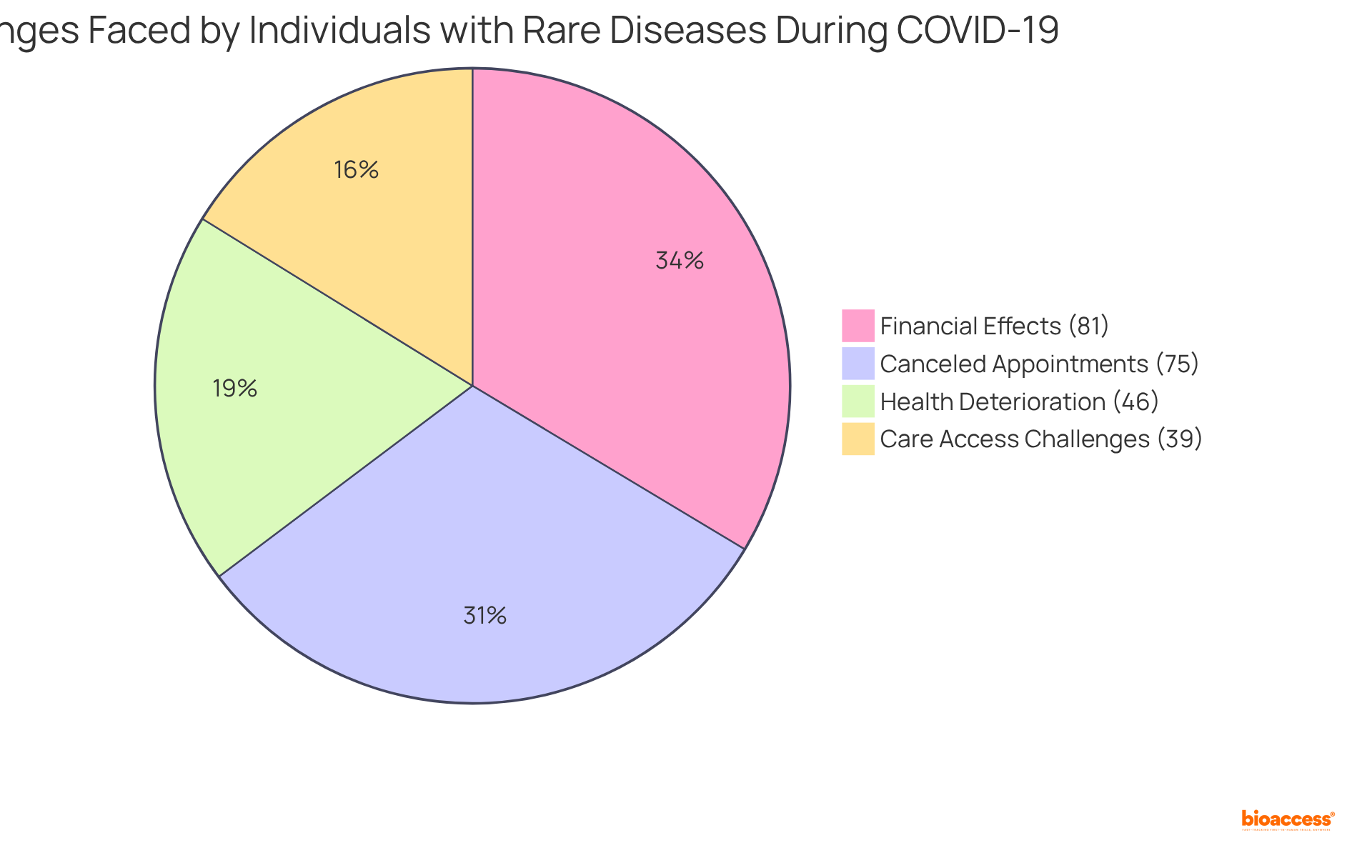
The COVID-19 pandemic has profoundly impacted individuals with rare disease and orphan disease, leading to significant disruptions in healthcare access and treatment continuity. Statistics indicate that nearly 39% of these individuals faced challenges in obtaining care due to pandemic-related limitations, while almost three-quarters of study participants reported canceled medical appointments. This disruption has resulted in postponed diagnoses and treatments, with many individuals experiencing a decline in their health condition. Notably, a significant 46% of individuals with uncommon health issues reported deteriorating health conditions during this period.
Furthermore, the transition to telemedicine, although providing some accessibility, has created difficulties in examination and communication, underscoring the necessity for a balanced approach to remote consultations. Healthcare experts have observed that the pandemic intensified existing weaknesses, highlighting the importance of prioritizing the needs of individuals with rare disease and orphan disease in future healthcare strategies and research efforts.
Moreover, 81% of individuals with uncommon health conditions reported financial effects due to the pandemic, illustrating the economic pressure on this group. The establishment of the ARDEnt coalition in response to these challenges represents a collaborative effort to address diagnostic delays and enhance support for individuals with rare disease and orphan disease. As we move forward, it is crucial to develop strategies that effectively address these challenges, ensuring that the unique needs of this population are met.
Future trends in studies of rare disease and orphan disease are poised for significant transformation due to technological advancements, enhanced collaboration, and a robust focus on patient-centered approaches. Innovations such as gene therapy and personalized medicine are at the forefront, with gene therapies showcasing remarkable progress; recent FDA approvals for therapies targeting sickle cell condition exemplify the potential of these treatments to revolutionize individual outcomes. Notably, approximately one-third of items in the global development pipeline are now directed toward uncommon condition indications, highlighting the growing recognition of these challenges.
Digital health solutions are emerging as critical instruments in the development of new therapies, facilitating real-time data collection and improving the quality of clinical trials. The integration of individual involvement and real-world evidence is increasingly vital, aiding in the prioritization of studies and ensuring that new treatments align with the authentic needs of those affected by uncommon conditions.
Organizations like bioaccess® are strategically equipped to navigate these evolving trends, harnessing their extensive experience and expertise to spearhead innovative projects. As the landscape of research on rare disease and orphan disease continues to progress, the focus on collaboration and technology is likely to yield more effective and accessible treatments for patients globally.
The exploration of strategies for advancing rare disease and orphan disease research reveals a pressing need for innovation and collaboration in this critical field. By understanding the unique challenges these conditions present, stakeholders can work together to enhance treatment options and improve patient outcomes. The integration of rapid clinical trials, regulatory navigation, and patient-centered approaches is essential for creating an effective framework that addresses the needs of those affected.
Key insights from the article highlight the importance of organizations like bioaccess® in accelerating clinical studies, the role of European Reference Networks in improving care, and the necessity of identifying unmet needs through patient engagement. Furthermore, the impact of COVID-19 has underscored the vulnerabilities faced by individuals with rare diseases, emphasizing the urgent need for adaptive strategies that prioritize their health and well-being.
Looking ahead, the future of rare disease research is bright, driven by technological advancements and a commitment to collaboration. By fostering partnerships and leveraging real-world data, the rare disease community can continue to innovate, ensuring that effective therapies reach those in need. It is crucial for all stakeholders to remain engaged and proactive in overcoming the barriers that persist, ultimately transforming the landscape of care for individuals with rare and orphan diseases.
What is bioaccess® and what does it specialize in?
bioaccess® specializes in accelerating clinical studies for rare diseases and orphan diseases by leveraging strategic sites across Latin America, the Balkans, and Australia.
How quickly can bioaccess® secure ethical approvals for clinical studies?
bioaccess® can secure ethical approvals in an impressive 4-6 weeks.
How does bioaccess®'s enrollment process compare to traditional markets?
The enrollment processes at bioaccess® are 50% faster than those in traditional markets.
Why is bioaccess®'s approach significant for innovators in the Medtech, Biopharma, and Radiopharma sectors?
This rapid approach expedites the delivery of treatments to the market and addresses the urgent needs of individuals affected by rare diseases, ensuring innovative therapies are accessible sooner.
Can you provide an example of a successful clinical trial conducted by bioaccess®?
The recent success of the SonoThrombectomy™ system in Chile exemplifies how swift clinical trials can lead to significant advancements in treatment options for venous thromboembolism.
What regulatory improvements have been made in Latin America regarding clinical trials?
Organizations like COFEPRIS have reduced review times for Phase III clinical trials to just 45 days, enhancing the region's attractiveness for conducting urgent clinical studies.
What are orphan diseases and why are they important to study?
Orphan diseases are rare conditions affecting fewer than 200,000 individuals in the U.S. They pose significant challenges due to their rarity and limited funding for research, impacting the quality of life for those affected.
What is the average time for an individual with a rare condition to receive a diagnosis?
The average time for an individual with a rare condition to receive a diagnosis is around five years.
What legislative support exists for research on rare diseases?
The Orphan Drug Act has facilitated the approval of over 880 orphan medications since its inception and is expected to drive increased funding for research in this area by 2025.
What are some challenges faced in rare disease research?
Challenges include stringent regulatory requirements, high development costs, and limited financial returns, which complicate the research and development of treatments.
How does bioaccess® assist clients in navigating regulatory challenges?
bioaccess® leverages its extensive knowledge of regulatory processes to provide ethical approvals quickly and facilitate faster enrollment in clinical trials.
What key services does bioaccess® offer to researchers?
Key services include feasibility studies, comprehensive project management for clinical trials, assistance with import permits, and timely reporting on study status and adverse events.