


The article centers on strategies for authorized agent Colombia Medtech pricing, underscoring the critical need to comprehend regulatory frameworks, market dynamics, and stakeholder engagement to optimize pricing strategies.
It illustrates how companies can effectively leverage local expertise, navigate INVIMA regulations, and adapt to emerging trends in value-based pricing.
This approach is essential for enhancing their competitive positioning within Colombia's expanding Medtech market.
The Colombian Medtech landscape is rapidly evolving, driven by a combination of regulatory changes, market dynamics, and technological advancements. As the sector anticipates significant growth, with projections indicating a market value of USD 4.13 billion by 2030, understanding the intricacies of authorized agent Colombia Medtech pricing becomes crucial for firms aiming to establish a competitive edge.
This article explores seven strategic approaches that Medtech companies can adopt to navigate the complexities of pricing in Colombia, ultimately ensuring their innovative solutions reach healthcare providers and patients efficiently.
What challenges and opportunities lie ahead for stakeholders in this dynamic environment? How can they leverage insights to optimize their pricing strategies?
bioaccess® spearheads clinical research in Colombia, facilitating efficient pathways for regulatory approvals and industry entry through authorized agent Colombia Medtech pricing for Medtech firms. By leveraging local expertise and a profound understanding of the Colombian healthcare landscape, bioaccess® utilizes an authorized agent colombia medtech pricing to accelerate clinical trials and significantly enhance competitive cost strategies. This approach not only expedites the research process but also aligns with the authorized agent colombia medtech pricing strategies, ensuring that innovative medical technologies are launched efficiently and effectively.
With the Colombian clinical trials sector projected to reach USD 257.9 million by 2030, alongside an anticipated compound annual growth rate (CAGR) of 6.8% from 2024 to 2030, the urgency for swift regulatory approvals has never been more critical. Successful case studies highlight how bioaccess® has empowered Medtech companies, as well as those relying on authorized agent colombia medtech pricing, to navigate the regulatory landscape, resulting in quicker market access and improved cost strategies that meet the needs of healthcare providers and patients alike.
As the landscape continues to evolve, bioaccess® stands as a pivotal partner for Medtech innovators striving to excel in Colombia's dynamic market.
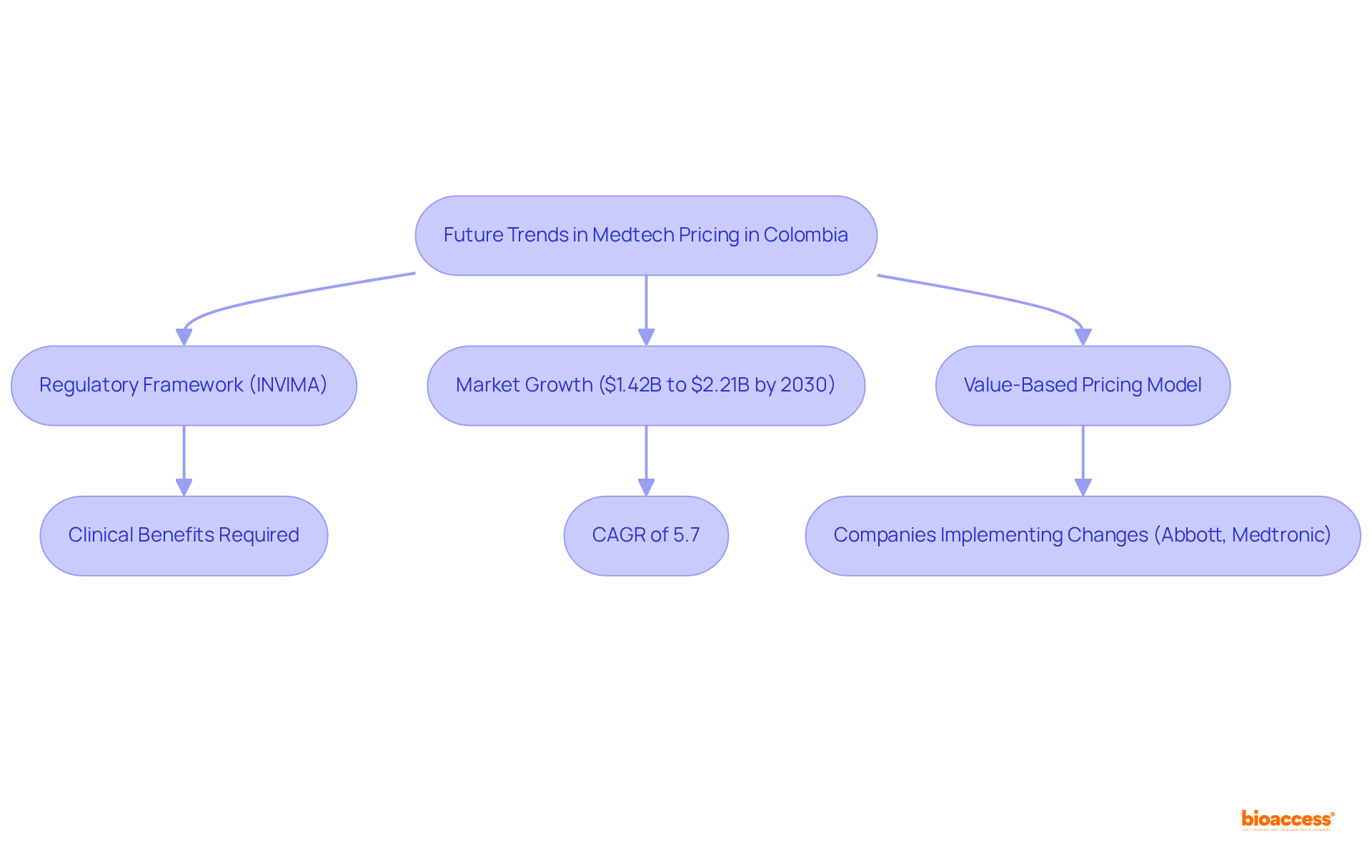
The Colombian regulatory system, overseen by INVIMA (Colombia National Food and Drug Surveillance Institute), is crucial in shaping the authorized agent Colombia medtech pricing strategies. Established in 1992 under the Ministry of Health and Social Protection, INVIMA is tasked with inspecting and supervising the marketing and manufacturing of health products, including medical devices. Its designation as a Level 4 health authority by the Pan American Health Organization/World Health Organization highlights its expertise in ensuring the safety, efficacy, and quality of medical technologies. Katherine Ruiz, a recognized authority in regulatory affairs for medical devices, underscores the necessity of comprehending these regulations.
Recent reforms have implemented value-based cost assessment (VBP) methodologies that evaluate the therapeutic value of medical devices prior to establishing authorized agent Colombia medtech pricing. This transition necessitates that companies articulate the clinical and economic benefits of their products clearly, thereby significantly impacting their market positioning and cost strategies. The revised cost structure requires that each indication of drugs with multiple uses be assessed individually, concentrating on the one with the least budget impact. As a result, businesses must remain vigilant regarding these evolving regulations to refine their cost structures and ensure compliance.
The Colombian Medical Technology market is anticipated to grow at a compound annual growth rate (CAGR) of 6.04% from 2025 to 2030, reaching an estimated US$4.13 billion by 2030, propelled by increased government investment and a rising demand for advanced healthcare solutions. This growth underscores the importance of adapting to the new VBP framework, which aims to enhance transparency and foster trust in pharmaceutical companies concerning the authorized agent Colombia medtech pricing. Stakeholders contend that the limited value set currently in place may underappreciate the true therapeutic value of technologies, potentially hindering patient access to vital treatments. Therefore, businesses must meticulously monitor these changes and adjust their launch plans accordingly to successfully navigate the new landscape.
To establish competitive costs within Colombia's Medtech sector, firms must formulate comprehensive access plans that account for authorized agent Colombia Medtech pricing and local health dynamics. Engaging with key stakeholders—including healthcare providers, payers, and regulatory bodies such as INVIMA—is essential for fostering product acceptance and securing reimbursement through authorized agent Colombia Medtech pricing. These factors significantly influence cost strategies.
As a Level 4 health authority recognized by PAHO/WHO, INVIMA serves as the authorized agent for Colombia MedTech pricing, playing a pivotal role in overseeing medical devices and ensuring compliance with safety and efficacy standards that can affect market entry timelines. By cultivating strong relationships with these stakeholders, firms can gain a deeper understanding of consumer needs and preferences, allowing for tailored strategies that reflect the unique characteristics of the Colombian market, particularly in terms of authorized agent Colombia Medtech pricing.
Notably, the Medical Technology sector in Colombia is projected to grow at a steady annual rate of 6.04% from 2025 to 2030, reaching an estimated US$4.13 billion by 2030. Furthermore, the medical devices sector was valued at USD 1.42 billion in 2022 and is expected to rise to USD 2.21 billion by 2030, demonstrating a CAGR of 5.7%. Leveraging bioaccess®'s expertise in navigating the regulatory landscape and reimbursement strategies empowers companies to optimize their market entry and cost models effectively. The increasing demand for advanced technological devices, such as robotic surgical systems and remote patient monitoring solutions, underscores the critical need for stakeholder engagement, particularly regarding authorized agent Colombia Medtech pricing in this dynamic market.

Public-private partnerships (PPPs) are fundamentally shaping the authorized agent Colombia Medtech pricing strategies. These collaborations between governmental organizations and private firms not only foster innovation but also significantly enhance access to medical technologies. For example, bioaccess™ has partnered with Welwaze Medical Inc. to facilitate the launch of the groundbreaking Celbrea® medical device, which aims to improve early detection of breast disease in Colombia. This collaboration underscores bioaccess™'s vital role in regulatory and market access consulting, adeptly navigating the complexities of the medical landscape.
By pooling resources and expertise, authorized agent Colombia Medtech pricing can effectively reduce costs, making medical devices more affordable for both medical providers and patients. Since the establishment of a legal framework for PPPs, Colombia has witnessed 45 initiatives submitted for funding, reflecting a strong commitment to harnessing private sector efficiency alongside public sector infrastructure.
The impact of these collaborations extends beyond mere cost reduction; they also facilitate the integration of innovative technologies into the healthcare system. With the health-tech sector projected to expand at a 39% compound annual growth rate, reaching US $50 billion by 2033, the importance of PPPs is increasingly pronounced. Medtech companies that actively pursue these partnerships can align their objectives with public health goals, ultimately reaping the benefits of shared resources and expertise.
Moreover, the success of initiatives such as the Centre for Image Guidance and Minimally Invasive Therapy (CIGMIT) illustrates the potential of targeted, small-scale PPPs in enhancing access to medical services. Since its inception in 2015, CIGMIT has catered to over 37,724 patients, demonstrating how strategic collaborations can lead to improved patient outcomes and profitability for involved stakeholders.
In conclusion, Medtech firms in Colombia should prioritize engagement in PPPs to leverage the advantages of shared innovation and cost reduction, which includes considerations for authorized agent Colombia Medtech pricing, thereby refining their financial strategies and contributing to a more equitable healthcare landscape. Furthermore, understanding the regulatory framework established by INVIMA, which oversees medical devices and ensures compliance with health standards, is crucial for successful market entry and sustainability.
The cost framework of clinical trials in Colombia, influenced by authorized agent Colombia Medtech pricing, is pivotal in shaping financial strategies. Trials conducted in this region can be 30% to 75% less expensive than those in North America or Europe, primarily due to lower labor costs and streamlined regulatory processes. Notably, the average assessment duration for clinical studies in Colombia is approximately 60 days—significantly quicker than many other regions.
This cost advantage not only empowers companies to price their products more competitively through authorized agent Colombia Medtech pricing but also enables them to allocate resources more effectively toward innovation and quality assurance. Medtech companies must scrutinize their clinical trial expenses and explore cost-saving strategies, such as:
By harnessing these benefits, companies can bolster their competitive positioning and drive growth in a challenging environment.
The competitive landscape within Colombia's Medtech sector significantly influences authorized agent Colombia Medtech pricing strategies. As an increasing number of businesses enter this arena, the pressure to maintain authorized agent Colombia Medtech pricing escalates. Medtech companies must conduct comprehensive assessments to understand their competitors' cost structures and value propositions in relation to authorized agent Colombia Medtech pricing. A pivotal element in this environment is INVIMA (Colombia National Food and Drug Surveillance Institute), which regulates the marketing and manufacturing of health products, including medical devices. INVIMA's classification as a Level 4 health authority by PAHO/WHO underscores its ability to ensure the safety, efficacy, and quality of medical devices, directly impacting economic dynamics and cost strategies.
In 2022, the Colombian medical devices sector was valued at USD 1.42 billion and is projected to reach USD 2.51 billion by 2025, growing at an annual rate of 6.52% until 2030. This expansion is fueled by a rising demand for advanced medical devices, bolstered by government policies and an aging population that heightens the prevalence of chronic diseases. By effectively differentiating their products and emphasizing unique advantages, companies can secure a competitive edge, allowing them to uphold their cost strategies. This approach is essential as consumer preferences increasingly lean towards innovative solutions, particularly in a market where expenditures on medical devices constitute approximately 5% of Colombia's total health spending, with overall medical expenditures nearing 9.0% of its GDP in 2021.
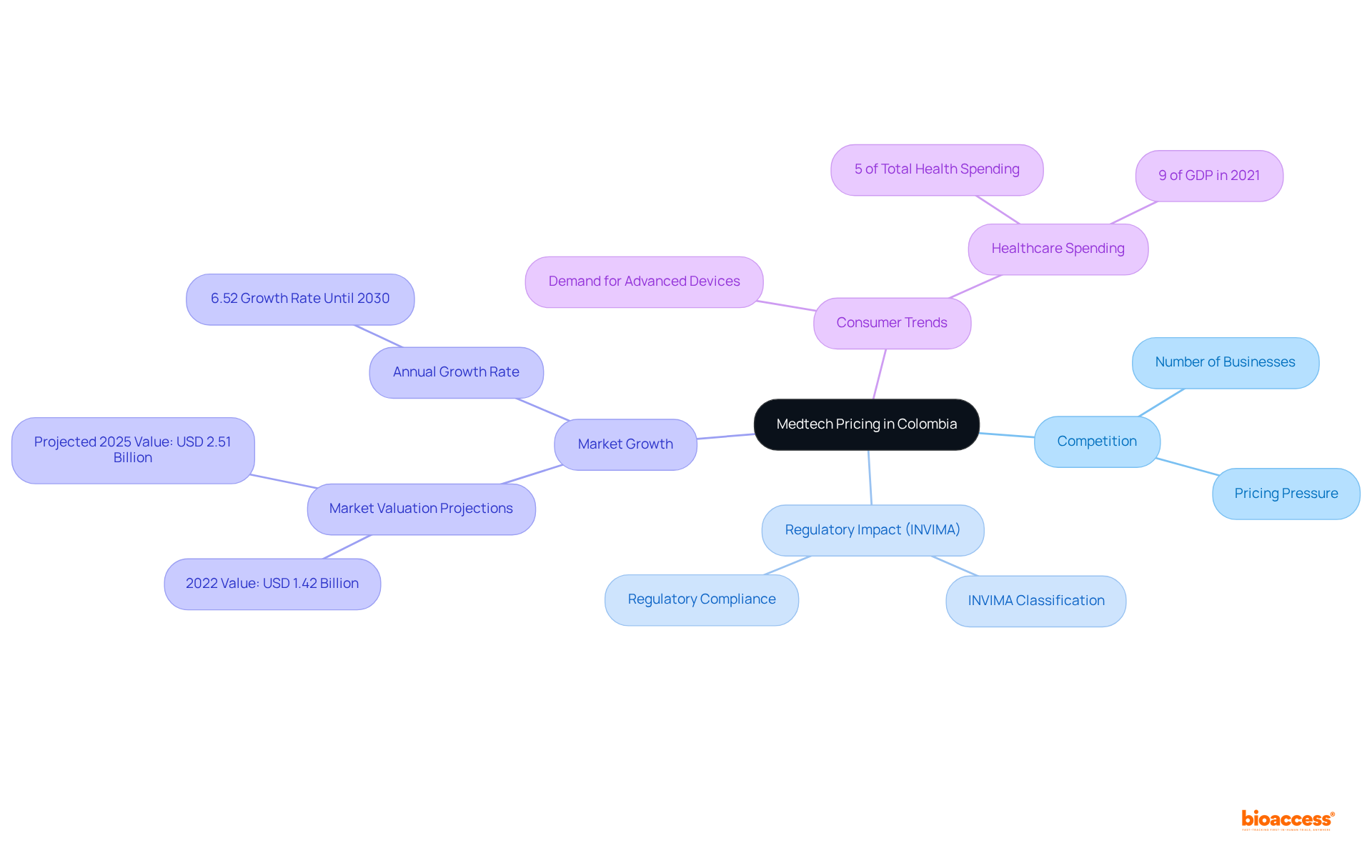
Economic elements, particularly inflation levels and medical expenditure, significantly influence authorized agent Colombia Medtech pricing strategies. As of early 2023, inflation reached an unprecedented 13.28%, compelling Medtech firms to reassess their pricing strategies, including the authorized agent Colombia Medtech pricing, to maintain competitiveness. With Colombia's medical spending per person projected to increase to $534.44 in 2023, up from $505.51 in 2022, substantial opportunities exist for Medtech companies to adapt their cost approaches and consider authorized agent Colombia Medtech pricing in response to rising medical expenditures.
To effectively navigate these economic conditions, companies must closely monitor key indicators, including:
By aligning their cost strategies with these market conditions, Medtech firms can enhance profitability while utilizing authorized agent Colombia Medtech pricing to ensure their products remain accessible to healthcare providers and patients alike.
Moreover, adapting to inflationary pressures requires innovative strategies, such as value-based cost models that highlight the clinical benefits of Medtech products, considering the authorized agent Colombia Medtech pricing. This approach can help justify price adjustments while maintaining a focus on patient outcomes. As the Colombian healthcare landscape evolves, Medtech firms must remain agile, leveraging insights from economic trends to refine their cost strategies for 2025 and beyond.
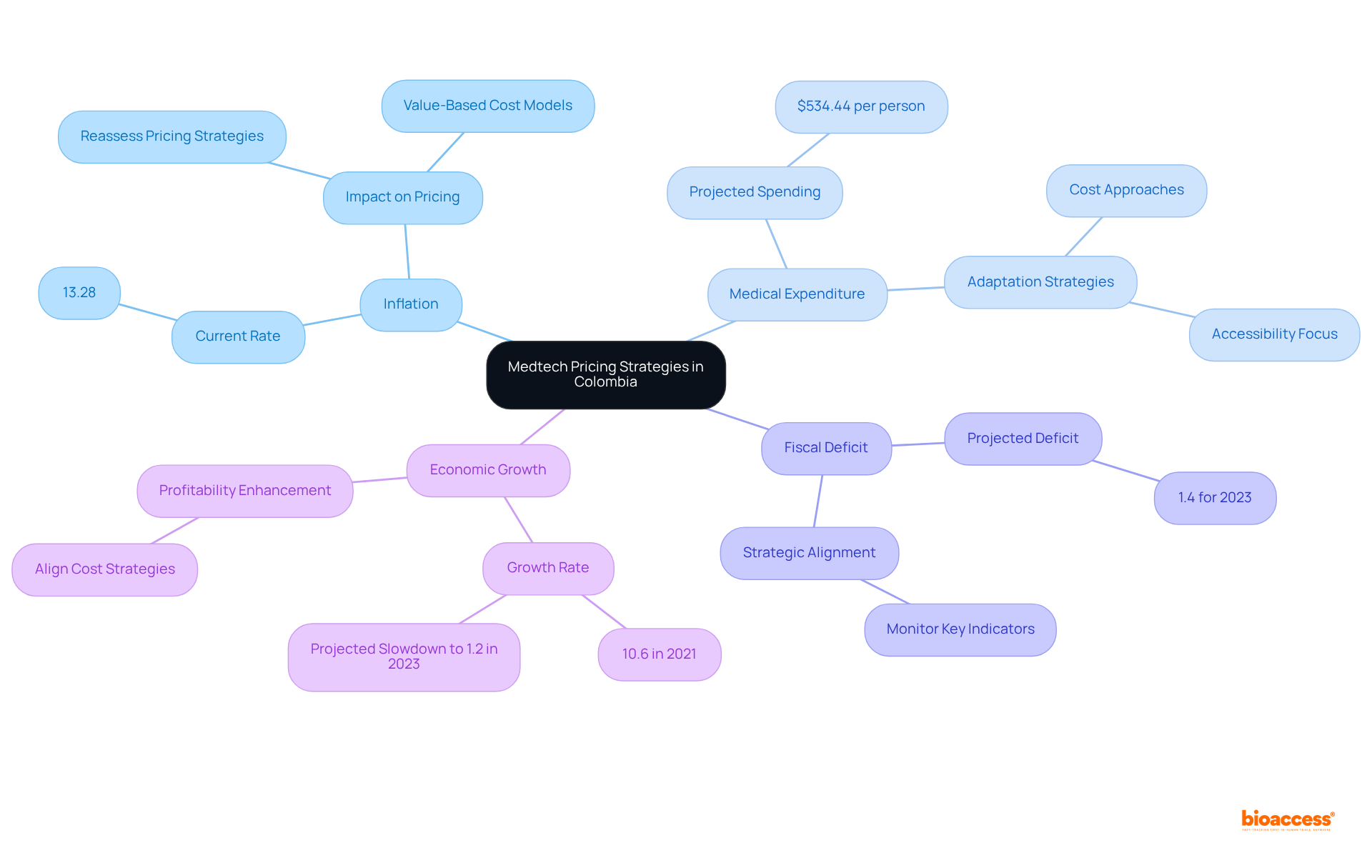
Technological advancements are fundamentally transforming the pricing strategies of Medtech in Colombia as managed by authorized agents. Innovations such as telemedicine and artificial intelligence (AI) not only enhance product offerings but also significantly improve patient outcomes. Companies that effectively leverage these technologies can justify premium pricing by demonstrating the added value they deliver. For instance, AI applications in medical settings have shown potential for reducing emergency room visits and hospitalizations by over 79 percent. This statistic underscores the efficiency gains that can be communicated to stakeholders. Moreover, the incorporation of telemedicine has enabled remote patient monitoring, allowing for more personalized care and prompt interventions, which are increasingly appreciated by both patients and medical providers.
As the healthcare landscape evolves, embracing these technological advancements allows companies to adopt more flexible pricing strategies that align with the authorized agent Colombia Medtech pricing, reflecting the growing demand for innovative solutions. The Colombian Medical Technology sector is anticipated to attain around US$3.08 billion by 2025, propelled by consumer preferences for advanced medical devices and digital health solutions. This shift enhances product competitiveness and positions companies to capitalize on the substantial opportunities presented by the market's growth. By coordinating cost approaches with the advantages of AI and telemedicine, Medtech companies can more effectively maneuver through the intricacies of the Colombian medical system and satisfy the demands of a more health-aware populace.
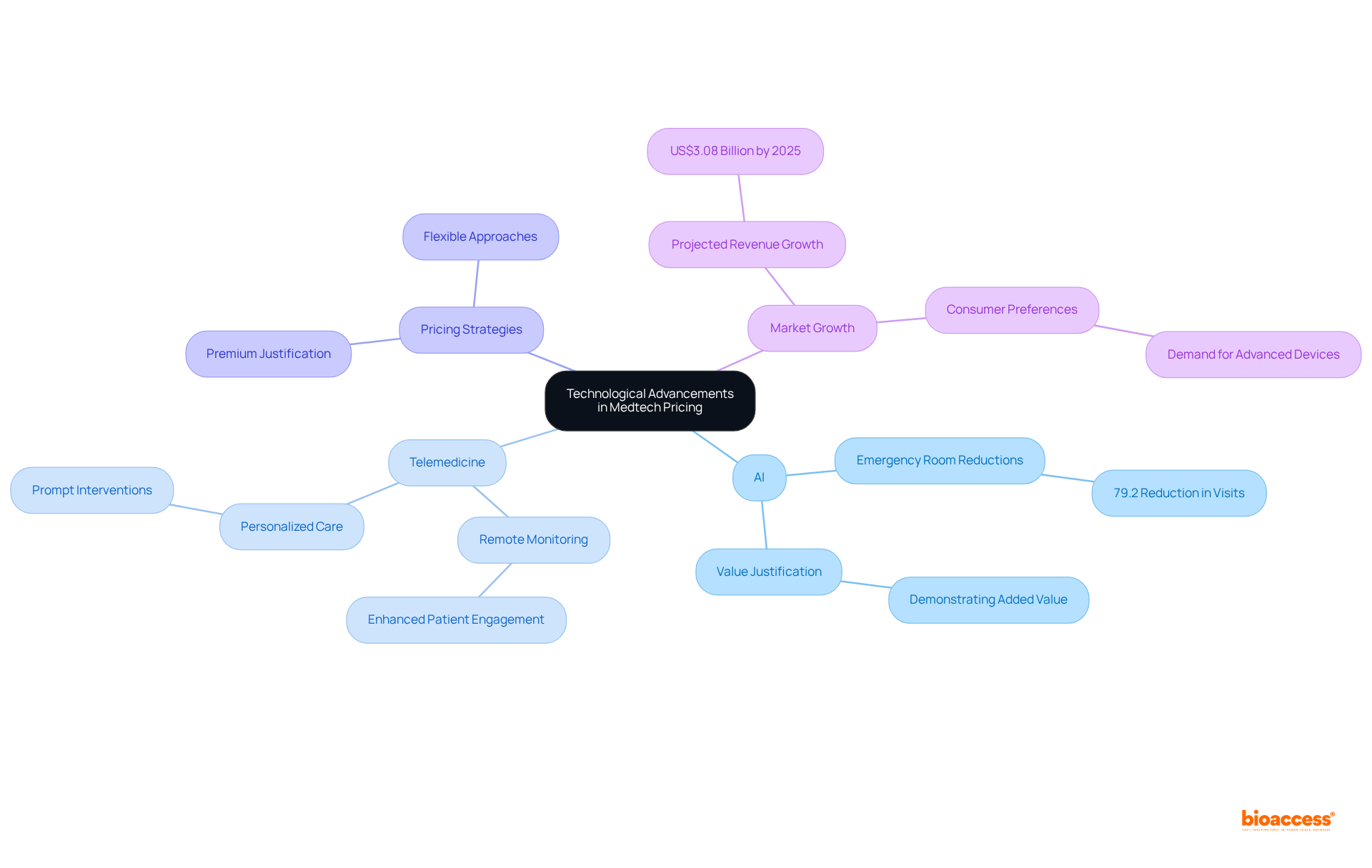
Effective stakeholder engagement is essential for Medtech companies aiming to optimize their authorized agent Colombia Medtech pricing. By collaborating with medical providers, payers, and patient advocacy groups, these companies can gain valuable insights into industry needs and preferences. In Colombia, successful partnerships have demonstrated that understanding the viewpoints of various stakeholders, particularly regarding authorized agent Colombia Medtech pricing, can significantly enhance product acceptance and ensure alignment with consumer expectations. For instance, firms that work closely with medical providers experience a marked improvement in cost management effectiveness, fostering greater trust and facilitating smoother market entry.
To effectively engage medical providers and payers, Medtech companies should focus on building robust connections through transparent communication about authorized agent Colombia Medtech pricing. Utilizing specific approaches, such as budget-impact models and value-based cost methods provided by bioaccess®, can further enhance these efforts. Additionally, incorporating Patient Access Mapping and Competitor Benchmarking can refine cost models to accurately reflect the evolving dynamics of the Colombian healthcare landscape as it progresses into 2025.
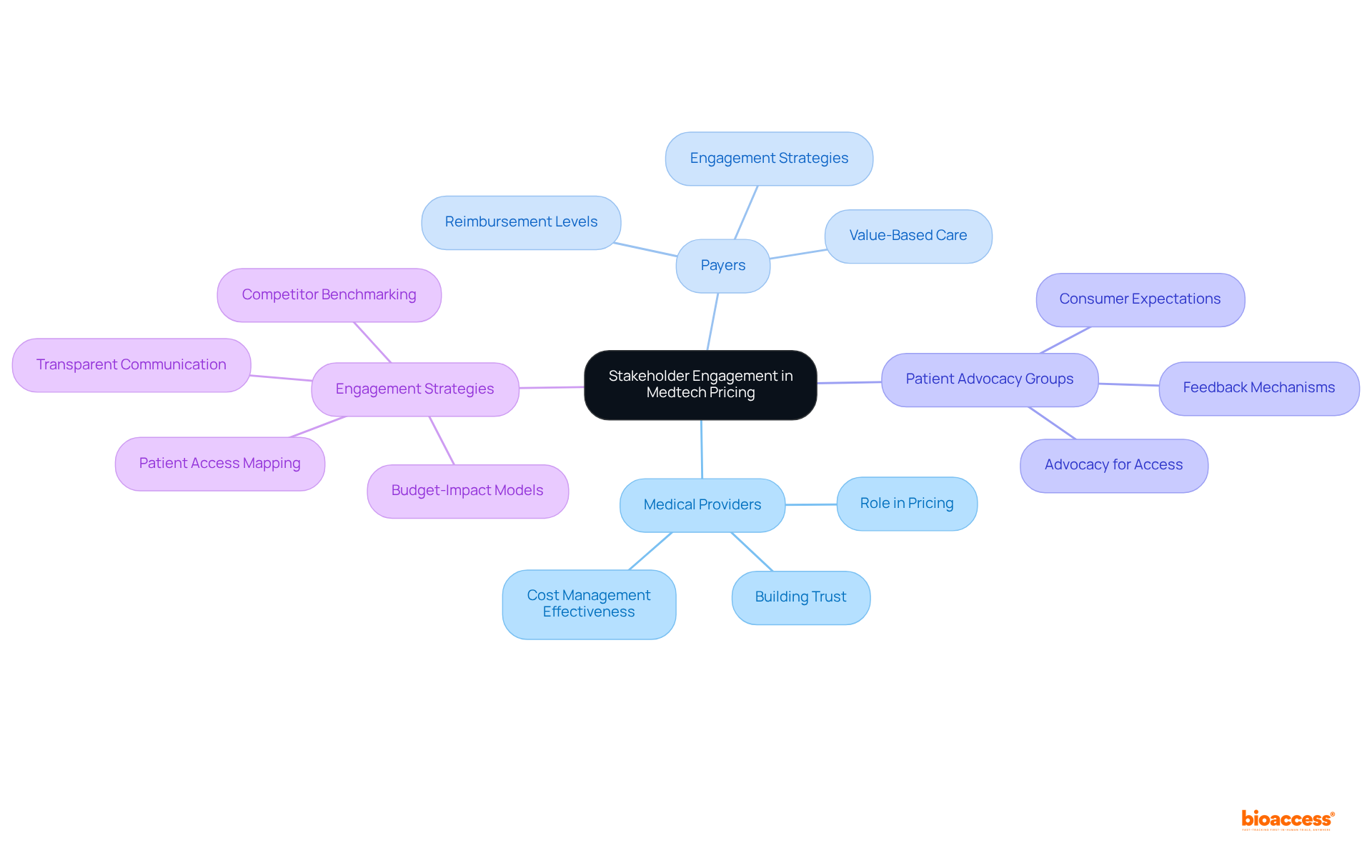
The Medtech environment in Colombia is undergoing significant transformation, with various emerging trends poised to influence authorized agent Colombia Medtech pricing. A notable shift toward value-based cost structures is gaining momentum, which enables authorized agent Colombia Medtech pricing to command higher prices for devices that demonstrably enhance patient outcomes. This strategy not only reflects the clinical advancements provided by medical devices but also aligns with the increasing regulatory scrutiny and the growing focus on patient-centered care.
Central to this regulatory framework is INVIMA (Colombia National Food and Drug Surveillance Institute), established in 1992 under the Ministry of Health and Social Protection. INVIMA plays a pivotal role in inspecting and supervising the marketing and manufacturing of health products, including medical devices. Its Directorate for Medical Devices and other Technologies ensures adherence to health standards and recommends technical standards for quality assurance. Recognized as a Level 4 health authority by the Pan American Health Organization/World Health Organization, INVIMA's oversight is crucial for ensuring the safety, efficacy, and quality of medical devices in Colombia.
As of 2022, the Colombian medical devices sector was valued at USD 1.42 billion and is projected to reach USD 2.21 billion by 2030, with a compound annual growth rate (CAGR) of 5.7%. This growth underscores the importance of adjusting financial strategies to meet evolving market demands. Companies such as Abbott and Medtronic are already implementing value-based cost models, which support premium pricing and establish themselves as authorized agents for Colombia Medtech pricing while reinforcing their roles as long-term partners in patient care. Their strategies are shaped by INVIMA's regulations, which stress the necessity for devices to demonstrate clear clinical benefits to justify higher prices.
In 2025, trends indicate that Medtech companies must continuously monitor industry developments and proactively adjust their cost structures. This agility will be essential for maintaining competitiveness in a swiftly evolving market. By leveraging advanced analytics and data-driven insights, companies can effectively segment customers and tailor cost strategies based on behavior and willingness to pay. Ultimately, the successful integration of value-based pricing will not only enhance profitability but also contribute to improved patient outcomes across Colombia's healthcare landscape.
The article underscores the pivotal role of authorized agent Colombia Medtech pricing strategies in shaping the future of medical technology within the country. By leveraging local expertise and adeptly navigating the regulatory landscape, Medtech firms can optimize their pricing models. This ensures that innovative solutions efficiently reach the market while remaining accessible to healthcare providers and patients.
Key insights highlight the necessity of comprehending the Colombian regulatory framework, the influence of public-private partnerships, and the importance of stakeholder engagement. Moreover, the article emphasizes how economic factors, technological advancements, and shifting market dynamics necessitate adaptive pricing strategies that align with value-based healthcare principles.
As Colombia's Medtech sector continues to expand, embracing these strategies will be essential for companies striving to thrive in a competitive landscape. Stakeholders must remain proactive in monitoring trends and adjusting their approaches to ensure sustainable growth and improved patient outcomes. By focusing on collaboration and innovation, Medtech firms can enhance their market presence and contribute to a more equitable healthcare system in Colombia.
What is bioaccess® and its role in clinical research in Colombia?
bioaccess® is a company that accelerates clinical research in Colombia by facilitating efficient pathways for regulatory approvals and industry entry through authorized agent Colombia Medtech pricing for Medtech firms. They leverage local expertise to enhance competitive cost strategies and expedite the research process.
What is the projected growth of the Colombian clinical trials sector?
The Colombian clinical trials sector is projected to reach USD 257.9 million by 2030, with an anticipated compound annual growth rate (CAGR) of 6.8% from 2024 to 2030.
How does the Colombian regulatory framework impact Medtech pricing?
The Colombian regulatory framework, overseen by INVIMA, shapes authorized agent Colombia Medtech pricing strategies. Recent reforms have introduced value-based cost assessment methodologies that evaluate the therapeutic value of medical devices, impacting market positioning and cost strategies.
What is INVIMA and its significance in the regulatory process?
INVIMA (Colombia National Food and Drug Surveillance Institute) is the regulatory body responsible for inspecting and supervising the marketing and manufacturing of health products, including medical devices. It is recognized as a Level 4 health authority by PAHO/WHO, ensuring the safety, efficacy, and quality of medical technologies.
What are the expected growth rates for the Colombian Medical Technology market?
The Colombian Medical Technology market is expected to grow at a CAGR of 6.04% from 2025 to 2030, reaching an estimated US$4.13 billion by 2030.
What strategies should firms adopt to ensure competitive pricing in Colombia's Medtech sector?
Firms should develop comprehensive access plans that consider authorized agent Colombia Medtech pricing and local health dynamics. Engaging with stakeholders such as healthcare providers, payers, and regulatory bodies is essential for product acceptance and securing reimbursement.
What are the implications of the new value-based pricing framework in Colombia?
The new value-based pricing framework requires companies to clearly articulate the clinical and economic benefits of their products, which significantly impacts their market positioning and cost strategies. It necessitates individual assessments for drugs with multiple uses, focusing on the indication with the least budget impact.
How can bioaccess® assist Medtech companies in Colombia?
bioaccess® assists Medtech companies by navigating the regulatory landscape and reimbursement strategies, enabling them to optimize their market entry and cost models effectively in Colombia's dynamic market.