


This article examines strategies for effectively managing treatment and control groups in clinical research, underscoring the critical role of robust methodologies in enhancing research outcomes. It articulates various approaches, including randomization, blinding, and ethical considerations, which together ensure the reliability and validity of clinical trials. Such methodologies are not merely procedural; they are essential for advancing patient care and propelling medical research forward. By implementing these strategies, researchers can foster a more trustworthy environment that ultimately benefits patients and the broader healthcare landscape.
The landscape of clinical research is evolving rapidly, making effective treatment and control group strategies increasingly vital for generating reliable data. As researchers strive to enhance the validity of their findings, it becomes essential to understand the nuances of randomization, blinding techniques, and ethical considerations. However, a significant challenge persists: how can researchers navigate the complexities of designing these groups to ensure robust outcomes that truly reflect the efficacy of interventions? This article delves into seven key strategies that can significantly improve the management of treatment and control groups, ultimately paving the way for advancements in medical research and patient care.
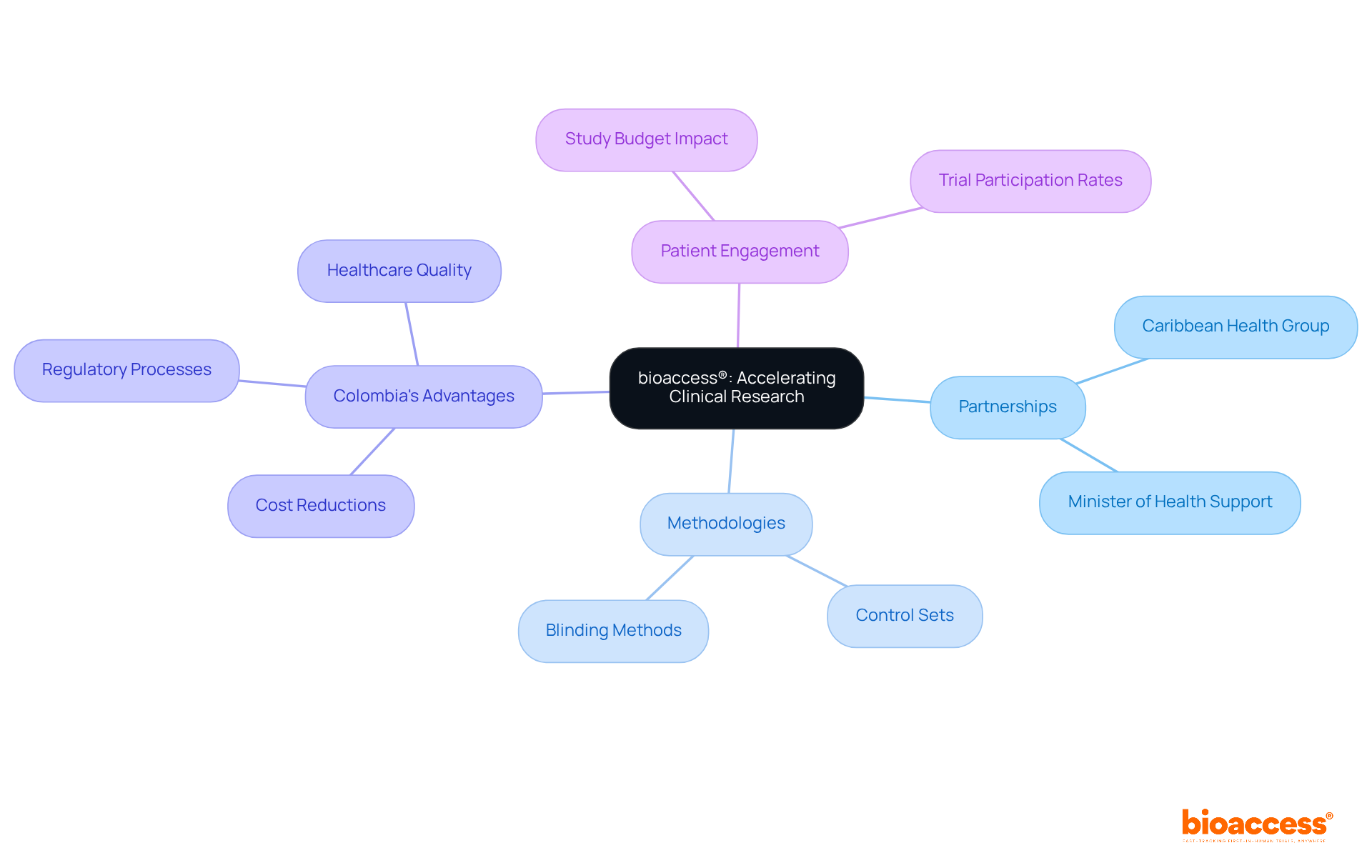
bioaccess® strategically leverages its presence in Latin America through a partnership with Caribbean Health Group, a collaboration prominently discussed during the meeting on March 29, 2019. This partnership aims to establish effective management and control strategies that significantly influence research outcomes. Supported by Colombia's Minister of Health, this initiative aspires to position Barranquilla as a leading hub for medical research in Latin America.
By capitalizing on regional regulatory efficiencies and diverse patient populations, bioaccess® accelerates research timelines, ensuring that the treatment and control groups are meticulously designed to produce valid and reliable results. This methodology not only enhances the quality of data collected but also facilitates a quicker transition from research to market, ultimately benefiting both innovators and patients.
Recent advancements in treatment and control groups, including the increasing application of multiple control sets and innovative blinding methods, further underscore bioaccess®'s commitment to excellence in research trials. Expert insights affirm that effective strategies for treatment and control groups are essential for demonstrating intervention efficacy, reinforcing bioaccess®'s status as a leader in advancing research.
Additionally, Colombia offers competitive advantages for first-in-human trials, such as cost reductions exceeding 30%, expedited regulatory processes with IRB/EC and MoH assessments taking only 90-120 days, and a high-quality healthcare system, all of which enhance the effectiveness of care and control strategies.
Furthermore, with research studies constituting approximately 40% of the pharmaceutical research budget in the United States, the financial implications of efficient study designs are substantial. The statistic that '47% of Americans who received invitations to participate in clinical trials confirmed their participation' exemplifies the effectiveness of treatment and control groups in enhancing patient engagement and recruitment.
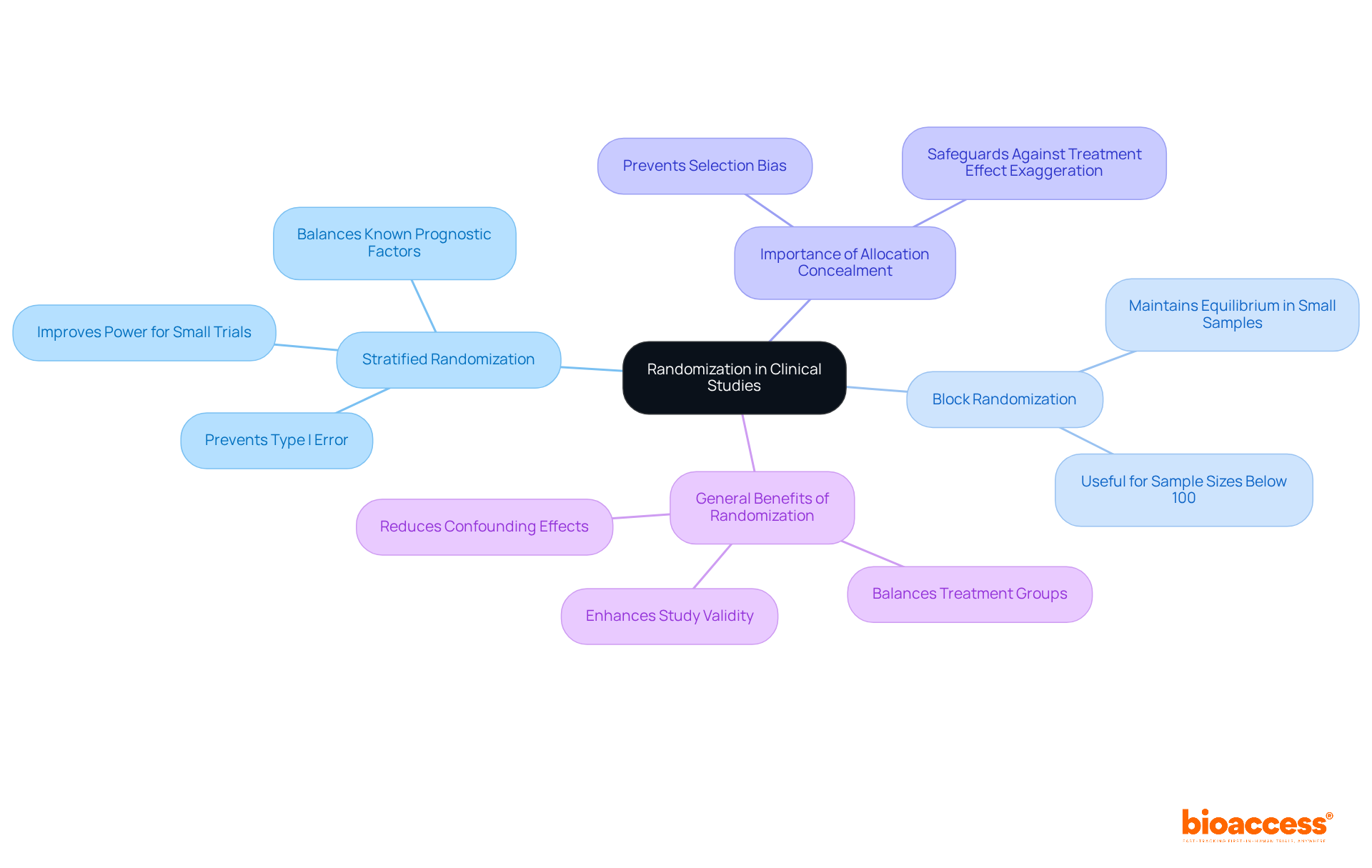
Randomization is a critical procedure that mitigates selection bias by ensuring each participant has an equal opportunity to be assigned to any treatment and control groups. This methodology is vital for ensuring the accuracy of comparisons between treatment and control groups, as it effectively balances both known and unknown confounding factors across different categories.
Implementing robust randomization techniques, such as:
Recent advancements in randomization techniques emphasize the importance of strict allocation concealment; as noted by The Lancet, "Proper randomisation relies on sufficient allocation concealment," which safeguards against biases that could compromise the integrity of the study. By adhering to these rigorous randomization methods, researchers can markedly improve the reliability and precision of their investigations.
In clinical studies, treatment and control groups are classified into several categories: placebo-controlled, active-controlled, and historical control sets. Each type plays a distinct role in research. Placebo-controlled cohorts are crucial for assessing the effectiveness of a new intervention by comparing it against a non-active measure, thereby isolating the effects of the intervention. For instance, in the Salk polio vaccine trials, the effectiveness of the vaccine was evaluated using both placebo and monitored control sets, leading to the conclusion that the vaccine was nearly 90% effective.
Conversely, active-controlled groups compare the new intervention to an existing standard therapy, providing a benchmark for evaluating relative efficacy. This approach is particularly useful in scenarios where a placebo may not be ethical, such as in life-threatening conditions. Recent studies have underscored the significance of active controls in demonstrating intervention superiority, as observed in the Diabetes Prevention Program, where lifestyle modifications and metformin were contrasted with a placebo.
While historical controls can offer valuable context, they often introduce biases due to differences in treatment protocols over time. For example, only 2 out of 63 studies in a recent analysis utilized historical controls, raising concerns about the validity of their findings. Notably, 63 studies (52%) included a comparative control, emphasizing the importance of control elements in medical research.
The selection of treatment and control groups is essential for the integrity of clinical studies. Researchers must consider the research question, study design, and ethical implications when selecting the appropriate control type. Current trends indicate a growing inclination for active-controlled trials, as they provide clearer insights into the efficacy of new solutions compared to existing options. Randomization is a vital technique to reduce bias and ensure comparability between treatment and control groups. Ultimately, the proper choice and execution of control sets are essential for ensuring the reliability and validity of research outcomes in healthcare. As Sarah Lee observed, "A control cohort is crucial to show that an intervention is better, less expensive, or linked to fewer complications in comparison to the standard procedure.

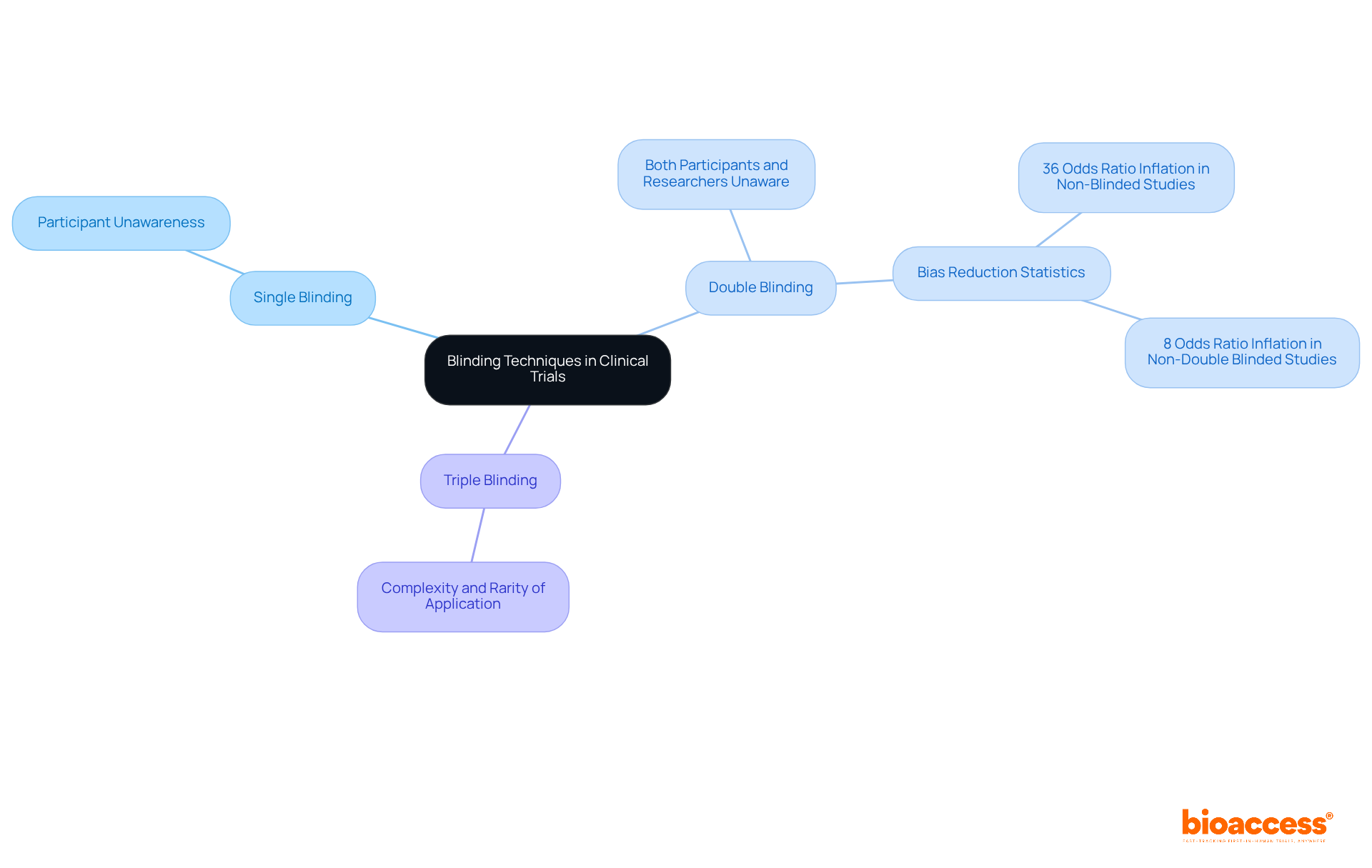
Blinding in clinical trials manifests in various forms, including single, double, or even triple blinding, contingent upon the study design. In single-blind studies, participants remain unaware of their group assignments, while double-blind studies guarantee that both participants and researchers are uninformed about intervention allocations. This dual-layer of blinding significantly mitigates the risk of bias in intervention administration and outcome reporting, thereby enhancing the integrity of the study results.
The significance of applying blinding methods cannot be overstated, as they are essential for accurately representing the true effectiveness of the intervention being evaluated. Studies indicate that blinding effectively diminishes the placebo effect, where participants may experience enhancements solely due to their belief in receiving care. For instance, a systematic review revealed that non-blinded outcome assessors inflated odds ratios by an average of 36%, while studies lacking double blinding demonstrated an overall 8% inflation of odds ratios. This underscores the necessity of blinding in minimizing bias.
Expert opinions further reinforce the value of blinding in treatment comparisons. Research has shown that double-blind studies are perceived as more credible and reliable by the scientific community and regulatory authorities. They eliminate observer bias, ensuring that researchers' knowledge does not influence their evaluations. Notably, blinding is employed in approximately 60% of research trials, although logistical challenges, such as those presented by specific interventions like surgical procedures or physical therapy, may occasionally obstruct its application.
Successful examples of double-blind studies illustrate the effectiveness of this approach in reducing bias. These studies consistently demonstrate that blinding leads to more dependable and valid outcomes, ultimately advancing medical knowledge and effectiveness. As the landscape of medical research evolves, the role of blinding remains a vital methodological safeguard, ensuring that the findings are both credible and applicable to real-world scenarios.
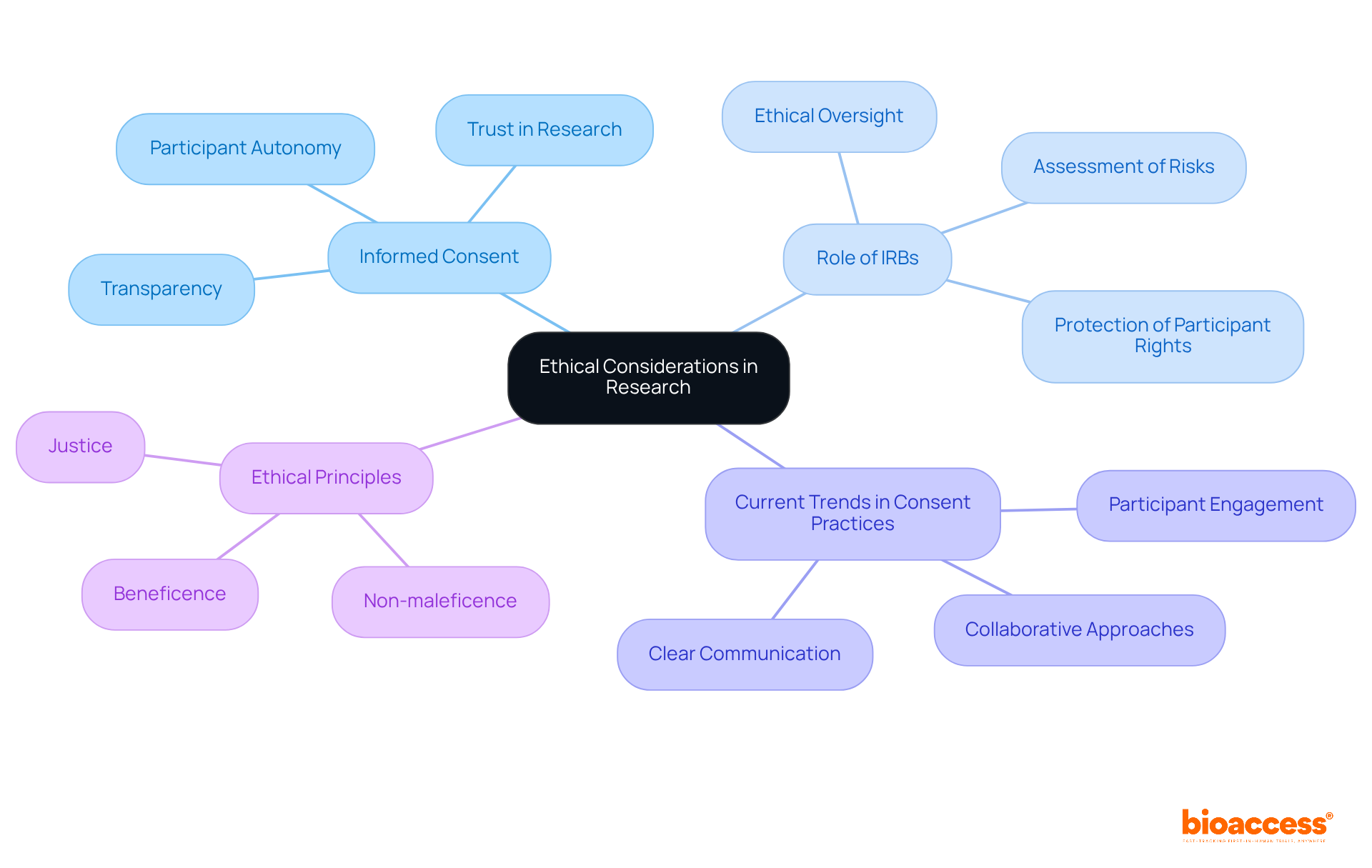
Adhering to ethical principles is paramount in clinical research, particularly concerning treatment and control groups. Obtaining informed consent stands as a fundamental requirement, ensuring that participants are thoroughly informed about the study's purpose, procedures, potential risks, and benefits. This process not only honors participant autonomy but also cultivates trust between researchers and participants.
The rationale for utilizing treatment and control groups is crucial, especially when it involves withholding care from participants. Ethical oversight by institutional review boards (IRBs) is instrumental in assessing the design and execution of studies, ensuring that participant rights and welfare are prioritized. For instance, in studies involving placebos, researchers must demonstrate that the potential benefits of the research outweigh the risks associated with withholding care.
Current trends in informed consent practices underscore the importance of transparency and participant engagement. Researchers are increasingly implementing strategies that involve participants in the consent process, fostering a more collaborative approach. This includes delivering clear, accessible information and ensuring that participants are aware of their rights, including the ability to withdraw from the study at any time.
The importance of informed consent in research involving participants cannot be overstated. It transcends mere regulatory compliance; it is a cornerstone of ethical research practice. As emphasized by the World Medical Association, the health and well-being of participants must always take precedence. Upholding these ethical standards not only safeguards participants but also bolsters the credibility and integrity of the research findings.
Creating effective treatment and control groups in research studies poses significant challenges, especially in terms of participant recruitment and retention, along with maintaining balanced group sizes. To address these pressing issues, several strategies can be employed:
Targeted Recruitment Methods: Utilizing demographic data and community outreach can effectively identify and engage specific populations that are often underrepresented in clinical trials. For instance, minorities frequently face barriers to involvement, with African Americans constituting 12% of the U.S. population yet only 5% of clinical study participants. Tailoring recruitment efforts to these communities not only enhances representation but also improves data accuracy.
Incentives for Participation: Offering financial compensation or other incentives can significantly boost recruitment rates. Research indicates that participant compensation is positively associated with recruitment success, particularly among economically disadvantaged populations. This strategy not only encourages enrollment but also fosters a sense of value for participants' time and contributions.
Adaptive Study Designs: Implementing adaptive study designs allows for modifications based on interim results, thereby enhancing participant retention. For example, if early data reveals a lack of efficacy, adjustments can be made to the treatment protocol, potentially increasing participant engagement and satisfaction.
Effective Communication: Clear and compassionate communication is paramount. Research shows that 92.7% of oncology patients deem it important to discuss studies with their doctors prior to participation. Establishing trust through open dialogue can alleviate fears and misconceptions about participation, particularly concerning the risks of receiving a placebo or adverse effects.
Logistical Support: Addressing logistical barriers is essential, especially for participants residing far from study centers. Approximately 70% of potential participants live more than two hours from a research location. Providing home visits or remote monitoring technologies can facilitate participation, particularly for individuals with chronic conditions who may experience mobility issues. A recent partnership between GlobalCare Clinical Trials and bioaccess™ exemplifies this approach, significantly reducing recruitment time by over 50% and achieving a retention rate exceeding 95%.
Utilizing Technology: Leveraging technology, such as artificial intelligence and machine learning, can streamline the recruitment process. A recent study demonstrated that AI could identify 16 potential participants in just one hour, compared to only two in six months using traditional methods. This efficiency can substantially reduce recruitment timelines and costs.
Retention Strategies: Once participants are enrolled, maintaining their involvement is critical. Strategies such as regular check-ins, personalized communication, and proactively addressing participant concerns can enhance retention rates. Studies reveal that 15% to 40% of enrolled participants drop out before completion, underscoring the necessity for effective retention plans.
By proactively addressing these challenges through targeted recruitment strategies, effective communication, and logistical support, research trials that include treatment and control groups can achieve more robust and reliable outcomes, ultimately advancing medical research and enhancing patient care.
The application of appropriate statistical techniques, such as t-tests and ANOVA, is essential for analyzing data from treatment and control groups in clinical research. T-tests effectively compare the means of two groups, while ANOVA is employed when assessing three or more groups, allowing researchers to determine if observed differences in outcomes are statistically significant. For instance, in studies evaluating the efficacy of a new medication, a t-test may be utilized to compare average recovery times between a treatment group and a control group, whereas ANOVA could be applied to investigate the effects of different dosages across multiple groups.
The significance of these statistical methodologies cannot be overstated; they form the backbone for deriving valid conclusions regarding the effectiveness of interventions. Recent advancements in statistical software tools, including R, SPSS, and SAS, have further improved the precision and efficiency of data analysis. These tools streamline complex calculations and visualizations, enabling researchers to concentrate on interpreting results and making informed decisions grounded in robust statistical evidence. Ultimately, comprehensive statistical analysis is vital for ensuring the reliability of findings in treatment and control groups studies, thereby advancing medical research and improving patient outcomes.
To ensure reliable outcomes in clinical research, researchers must standardize treatment protocols and data collection methods across all sites and participants. This standardization necessitates:
By promoting consistency, researchers can significantly reduce variability, thereby enhancing the credibility of study outcomes and increasing their relevance to diverse populations. For instance, implementing a core outcome set (COS) simplifies the evaluation process, ensuring that all studies assess the same essential outcomes, which is crucial for comparing findings across various studies.
Furthermore, regular refresher training sessions can reinforce adherence principles among research personnel, ultimately leading to improved data integrity and participant safety. The commitment to standardized protocols not only safeguards the quality of the research but also fosters trust with stakeholders and participants alike.
The results of therapy and treatment and control groups studies provide valuable insights into the effectiveness of interventions, guiding future research designs. For instance, ReGelTec's Early Feasibility Study on HYDRAFIL™ in Colombia not only demonstrated successful treatment outcomes for chronic low back pain but also highlighted the advantages of conducting studies in this domain, achieving over a 50% reduction in recruitment time and 95% retention rates.
By analyzing patterns and results from such studies, researchers can pinpoint areas for enhancement, refine methodologies, and formulate new hypotheses. This iterative process is crucial for advancing medical knowledge and enhancing patient care, particularly as organizations like bioaccess® leverage their expertise in managing trials to optimize patient recruitment and streamline processes.
The impact of these medical studies transcends individual findings, contributing to local economies through job creation and healthcare improvements, thus fostering international collaboration in medical research.
To maximize the effectiveness of treatment and control groups in clinical research, it is crucial for researchers to prioritize best practices like meticulous planning and fostering clear communication within their teams. Involving stakeholders from the outset is crucial; this approach not only fosters transparency but also ensures compliance with ethical standards throughout the research process. Continuous observation of the experiment's progress allows for prompt modifications, thereby guaranteeing that the study remains aligned with its objectives. By embracing these strategies, researchers can significantly enhance the quality and reliability of their clinical trials, ultimately leading to improved patient outcomes. Current trends underscore the rising importance of stakeholder engagement, emphasizing collaborative approaches that integrate diverse perspectives, which enrich the research process and drive innovation.
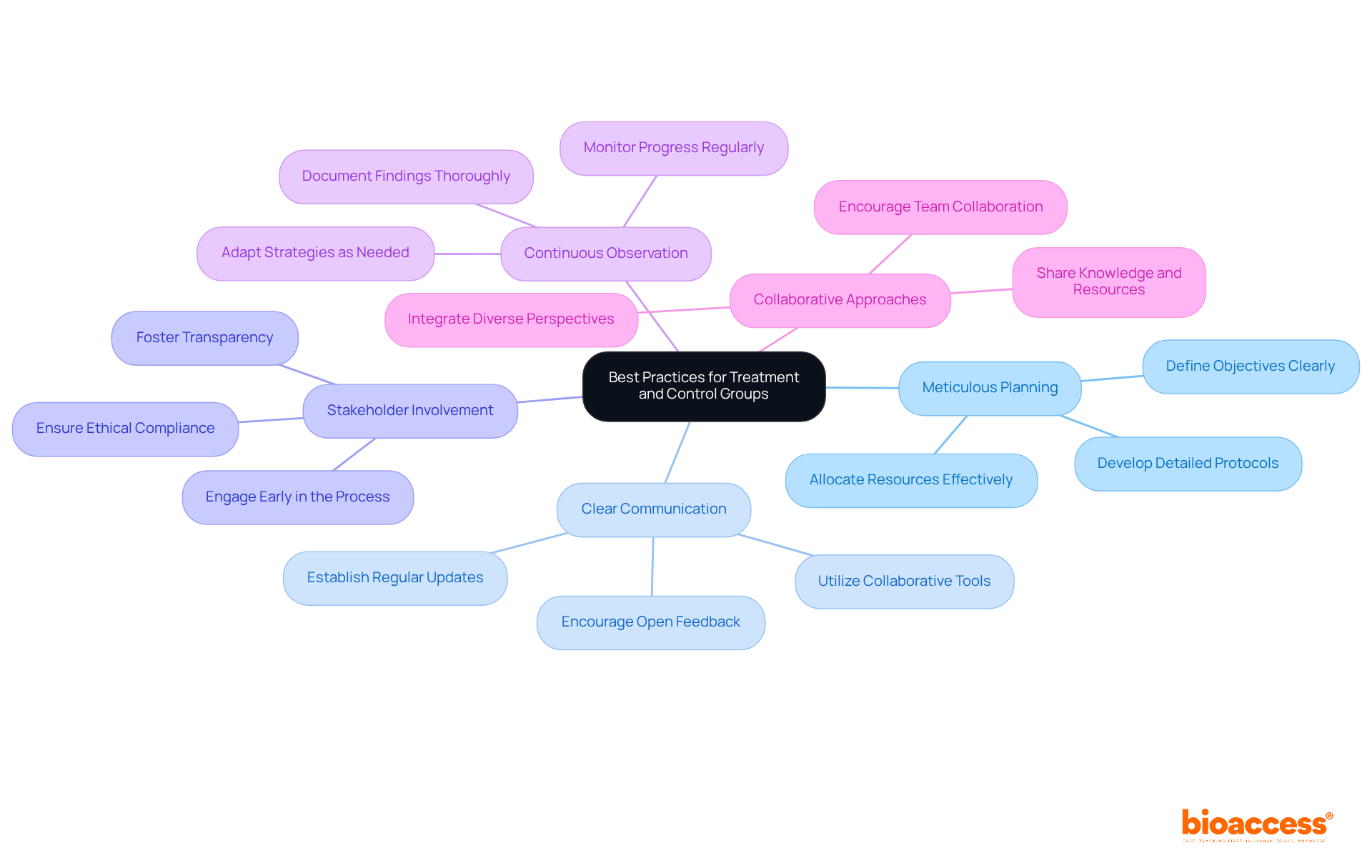
The exploration of effective treatment and control group strategies underscores the pivotal role these elements play in enhancing the credibility and reliability of clinical research. By implementing robust methodologies, such as randomization and blinding, researchers can significantly mitigate biases that may skew results, ultimately leading to more valid conclusions regarding the efficacy of interventions.
Key insights from the article emphasize the importance of thoughtful design and execution of treatment and control groups. From utilizing diverse control types to ensure ethical standards are met to leveraging advanced statistical methods for data analysis, each strategy contributes to the overarching goal of improving patient outcomes and advancing medical knowledge. The focus on ethical considerations and participant engagement further reinforces the integrity of research practices.
As the landscape of clinical research evolves, insights gained from treatment and control group studies will be instrumental in shaping future investigations. Researchers are encouraged to adopt best practices that prioritize transparency, stakeholder involvement, and adaptive methodologies. By doing so, they will not only enhance the quality of their trials but also foster a more inclusive and effective research environment that ultimately benefits patients and the broader healthcare community.
What is bioaccess® and what is its primary goal in clinical research?
bioaccess® is an organization that aims to accelerate clinical research by establishing effective treatment and control group strategies, particularly in Latin America, through its partnership with Caribbean Health Group.
How does bioaccess® leverage its partnership with Caribbean Health Group?
The partnership focuses on implementing effective management and control strategies that influence research outcomes, with the goal of positioning Barranquilla as a leading hub for medical research in Latin America.
What advantages does Colombia offer for clinical research, according to bioaccess®?
Colombia provides competitive advantages such as cost reductions exceeding 30%, expedited regulatory processes that take only 90-120 days, and a high-quality healthcare system, which enhance the effectiveness of care and control strategies.
Why is randomization important in clinical trials?
Randomization is crucial as it mitigates selection bias by ensuring each participant has an equal chance of being assigned to any treatment or control group, thereby improving the accuracy of comparisons and balancing confounding factors.
What are some robust randomization techniques mentioned in the article?
The article mentions stratified randomization, which is useful for small studies, and block randomization, which helps maintain equilibrium among treatment groups when the sample size is below 100 subjects.
What types of control groups are used in clinical trials?
Control groups in clinical trials can be classified as placebo-controlled, active-controlled, and historical control sets, each serving a distinct purpose in assessing the effectiveness of new interventions.
How do placebo-controlled groups function in clinical studies?
Placebo-controlled groups compare a new intervention against a non-active measure to isolate the effects of the intervention, which is essential for assessing its effectiveness.
What is the role of active-controlled groups in clinical research?
Active-controlled groups compare a new intervention to an existing standard therapy, providing a benchmark for evaluating relative efficacy, especially when a placebo may not be ethical.
What concerns are associated with historical control groups?
Historical control groups may introduce biases due to differences in treatment protocols over time, which can affect the validity of the findings.
Why is the selection of treatment and control groups critical in clinical studies?
The selection is essential for maintaining the integrity of clinical studies, as it impacts the reliability and validity of research outcomes, and researchers must consider the research question, study design, and ethical implications when choosing control types.