


The article outlines seven strategies to enhance the delivery and efficacy of siRNA therapeutics, emphasizing the importance of overcoming both extracellular and intracellular barriers. By utilizing advanced delivery systems and incorporating nucleotide modifications, these strategies are pivotal in advancing RNA-based treatments. Each approach, including the use of lipid nanoparticles and cell-penetrating peptides, is substantiated by evidence that demonstrates improved stability, cellular uptake, and therapeutic effectiveness. This highlights their critical roles in the evolution of siRNA therapeutics, underscoring the necessity for innovative solutions in clinical research.
The landscape of RNA therapeutics is rapidly evolving, with small interfering RNA (siRNA) at the forefront of innovative treatment strategies. As the demand for effective delivery mechanisms intensifies, researchers and biopharmaceutical companies are actively exploring a variety of advanced methods to enhance the efficacy of siRNA therapeutics. This article delves into seven cutting-edge strategies:
These strategies promise to overcome significant delivery challenges and maximize therapeutic potential. What are the implications of these advancements for the future of gene therapy? How can they reshape the treatment landscape for various diseases? These questions are pivotal as we navigate the promising terrain of RNA therapeutics.
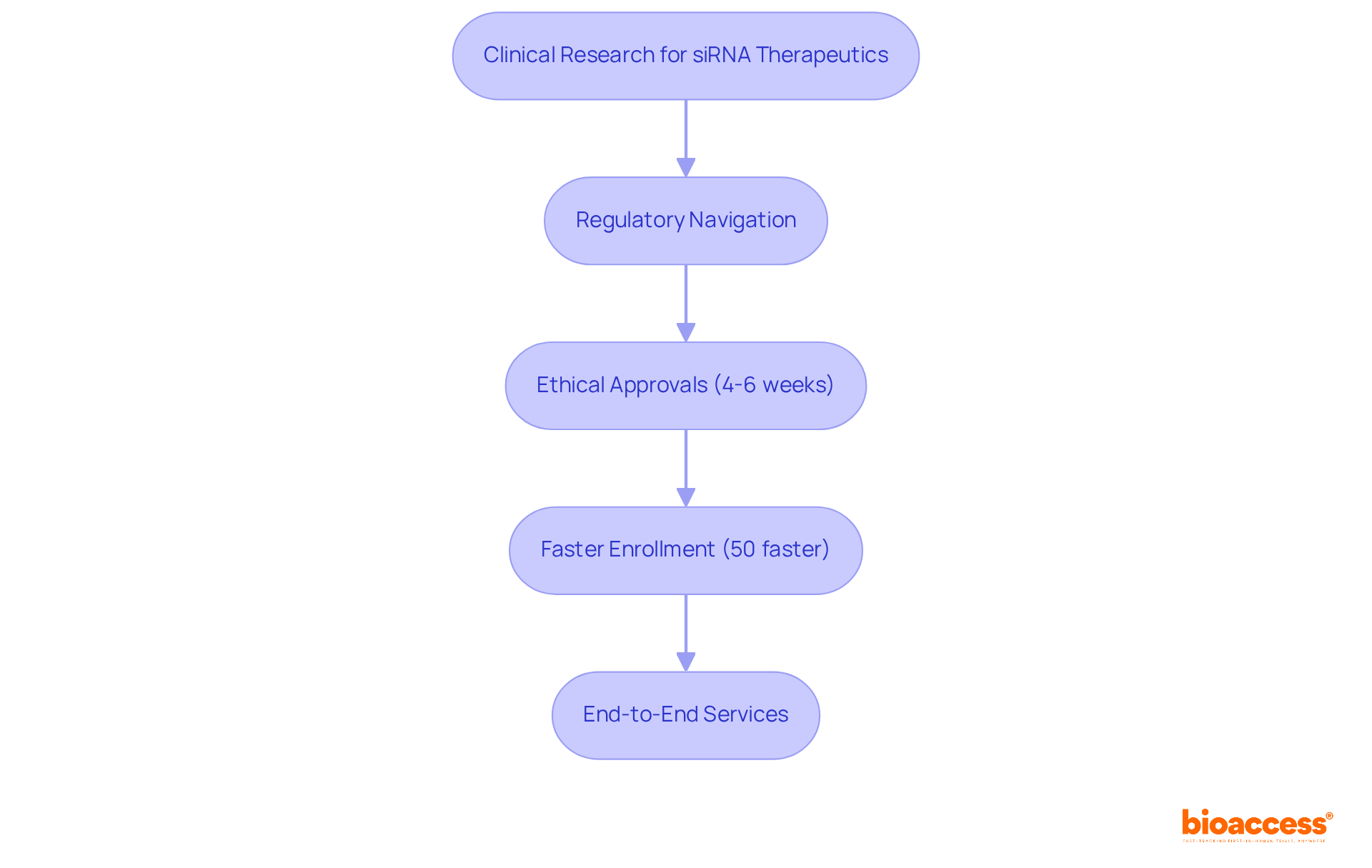
bioaccess® excels in expediting clinical research for sirna therapeutics by effectively navigating the regulatory landscapes of Latin America, the Balkans, and Australia. With ethical approvals secured in a remarkable 4-6 weeks and enrollment processes that are 50% faster than traditional markets, bioaccess® offers a significant strategic advantage for Medtech, Biopharma, and Radiopharma innovators.
Our end-to-end services include comprehensive clinical trial management, from feasibility studies and site selection to compliance reviews and project management, ensuring that every aspect of the trial is handled with expertise and efficiency. This agility is essential for companies looking to capitalize on the rapidly evolving area of RNA treatments, particularly in sirna therapeutics, allowing them to introduce their innovations to the market quickly and effectively.
The effect of regulatory speed on clinical trial success rates cannot be exaggerated; industry leaders consistently highlight that streamlined processes are essential for maintaining competitiveness in the changing treatment landscape. By collaborating with bioaccess®, companies can improve their likelihood of success in clinical trials, especially in the rapidly growing field of sirna therapeutics.
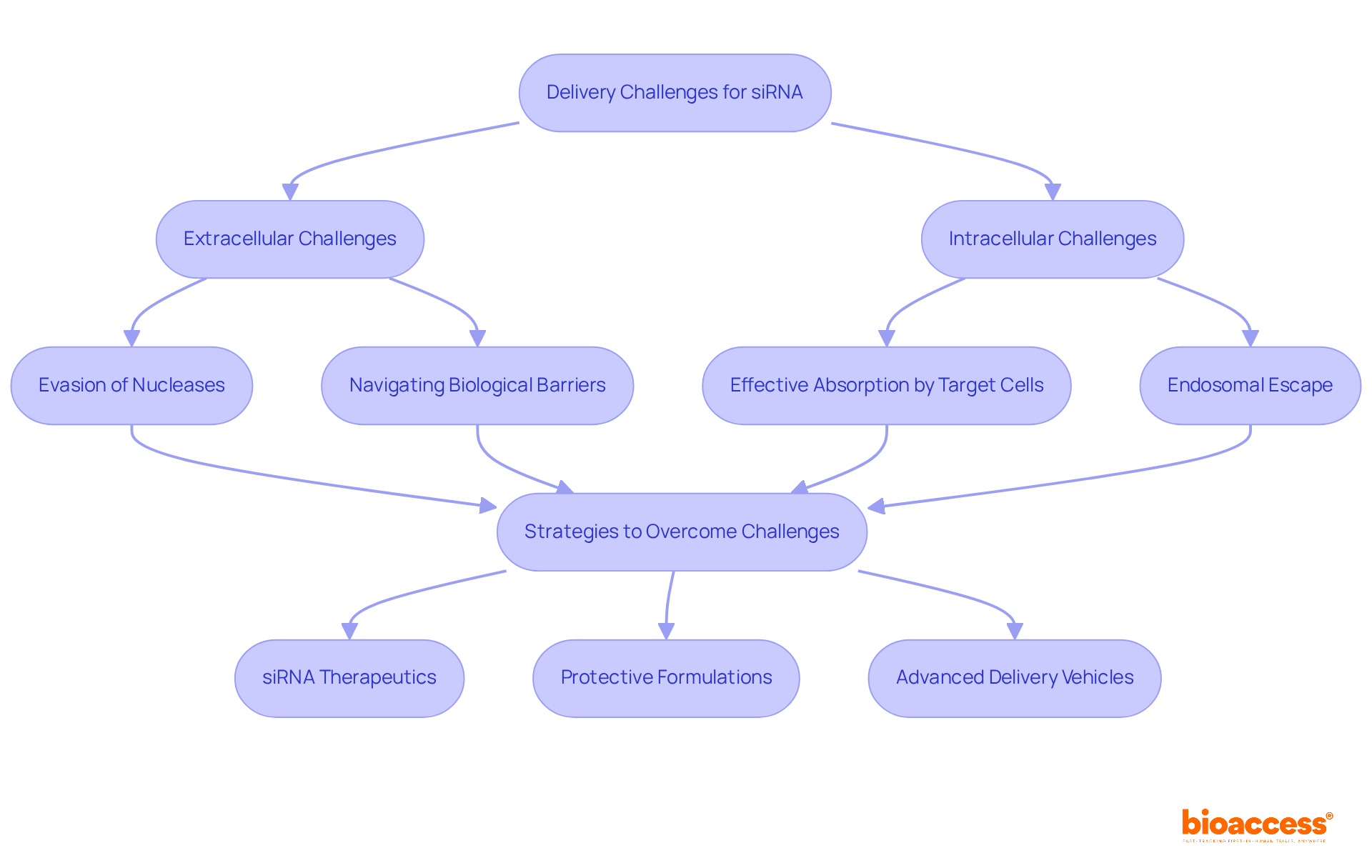
The efficient administration of sirna therapeutics encounters substantial extracellular and intracellular hurdles. Extracellularly, small interfering RNA must evade degradation by nucleases and navigate through biological barriers, such as the endothelial lining of blood vessels. Within cells, it is crucial for small interfering RNA to be effectively absorbed by target cells and evade endosomal compartments to access the cytoplasm, where it can exert its beneficial effects. To address these challenges, strategies such as:
are employed to enhance cellular uptake and facilitate endosomal escape.
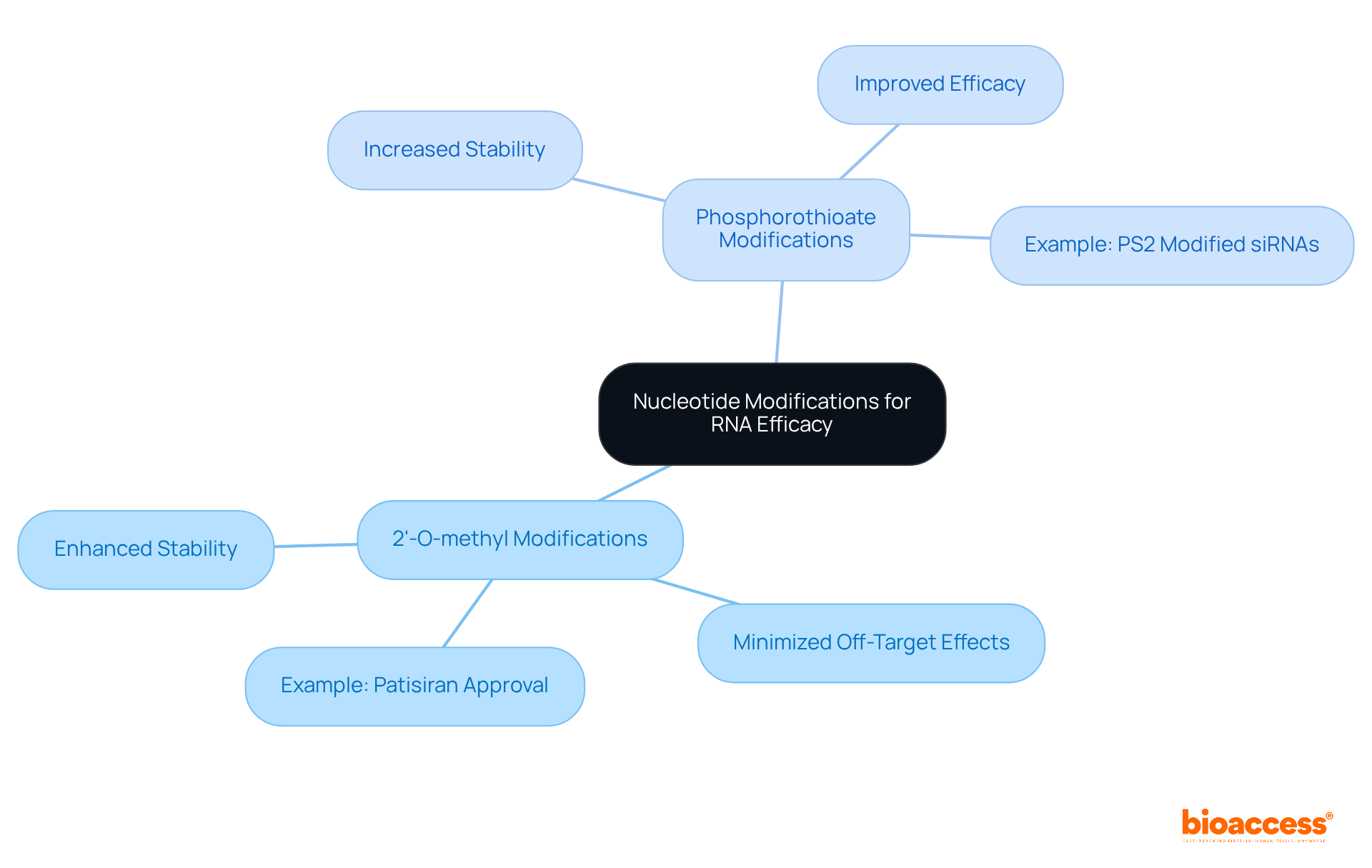
Nucleotide modifications, particularly 2'-O-methyl and phosphorothioate changes, are crucial for enhancing the stability and effectiveness of RNA-based treatments. These modifications not only improve resistance to enzymatic degradation but also minimize off-target effects, thereby expanding the treatment window.
For instance, research has shown that small interfering RNAs with complete 2'-O-methyl modifications can achieve target mRNA reduction comparable to unmodified versions. This is exemplified by the approval of patisiran, the first small interfering RNA treatment, which received regulatory authorization in 2018.
Furthermore, phosphorothioate alterations have demonstrated increased stability and effectiveness, with studies indicating that phosphorodithioate (PS2) modified small interfering RNA outperforms its unmodified counterparts.
As the field progresses, it is essential to integrate these modifications during the design phase of sirna therapeutics to enhance their treatment potential and ensure effective gene silencing. Experts emphasize that the careful selection of modifications in sirna therapeutics can significantly influence the efficacy of RNA interference treatments, paving the way for advancements in precision medicine across various disease areas.
However, it is equally important to acknowledge the challenges associated with RNA interference administration, necessitating tailored strategies to optimize the effectiveness of these innovative treatments.
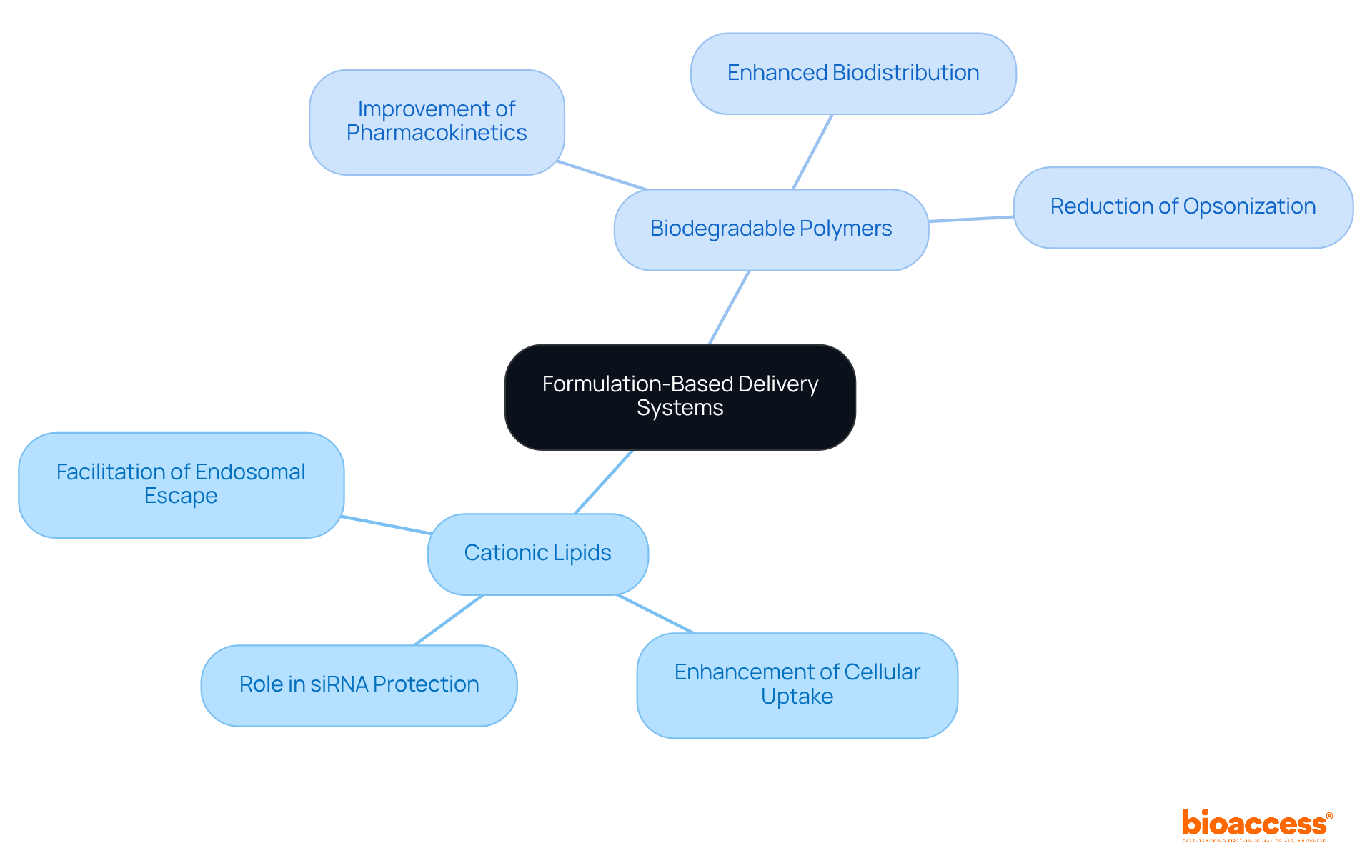
Formulation-based delivery systems, such as lipid nanoparticles (LNPs) and polymeric carriers, are pivotal in the effective delivery of siRNA therapeutics for RNA interference treatments. These systems not only protect small interfering RNA from degradation but also enhance cellular uptake and facilitate endosomal escape. It is essential for researchers to explore various formulations, including:
to improve the pharmacokinetics and biodistribution of siRNA therapeutics, ultimately leading to better treatment outcomes.
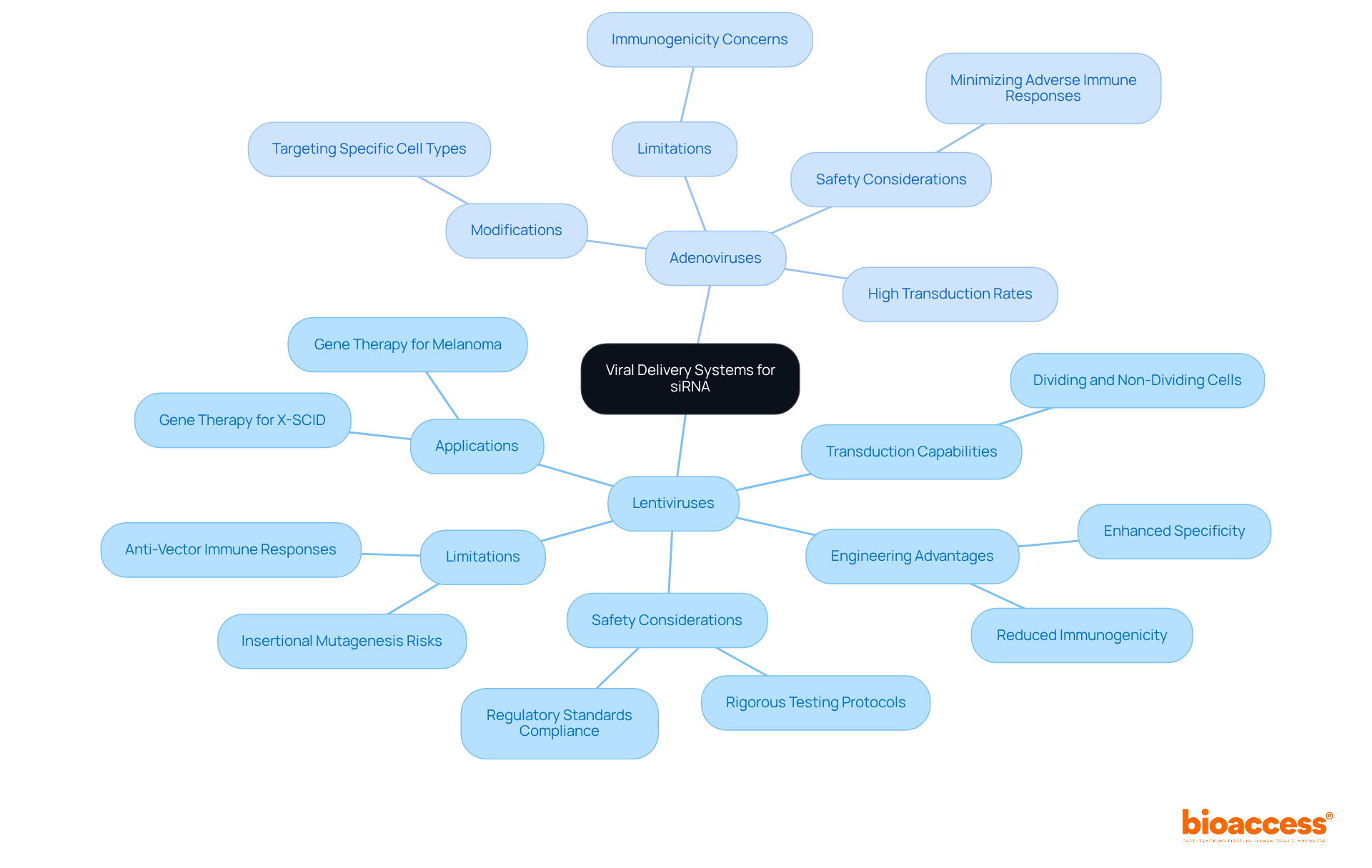
Viral delivery mechanisms, particularly lentiviruses and adenoviruses, play a pivotal role in siRNA delivery due to their exceptional transfection efficiency and capacity to introduce genetic material into target cells. Lentiviruses, for instance, are capable of transducing both dividing and non-dividing cell types, rendering them versatile for a multitude of therapeutic applications. Their engineering permits enhanced specificity and a reduction in immunogenicity, which is essential for minimizing adverse immune responses. As highlighted by Brown et al., a significant limitation of viral-based gene therapy lies in the anti-vector and anti-transgene immune responses, which can lead to the elimination of corrected entities by the immune system. Conversely, adenoviruses are recognized for their high transduction rates and can be modified to effectively target specific cell types.
Successful applications of these vectors are evident in gene therapy trials for conditions such as melanoma and X-SCID, where lentiviral vectors have demonstrated significant treatment effectiveness. Furthermore, adenoviral vectors have been instrumental in advancing gene therapy, contributing to the approval of treatments for various genetic disorders.
However, the deployment of these viral vectors requires careful consideration of safety and regulatory aspects. Current safety protocols emphasize minimizing the risk of insertional mutagenesis and immune responses, which have historically presented challenges in gene therapy. Experts in the field underscore the importance of rigorous testing and adherence to regulatory standards to ensure the secure application of these vectors in clinical settings, thereby fostering trust in their use for siRNA therapeutics.
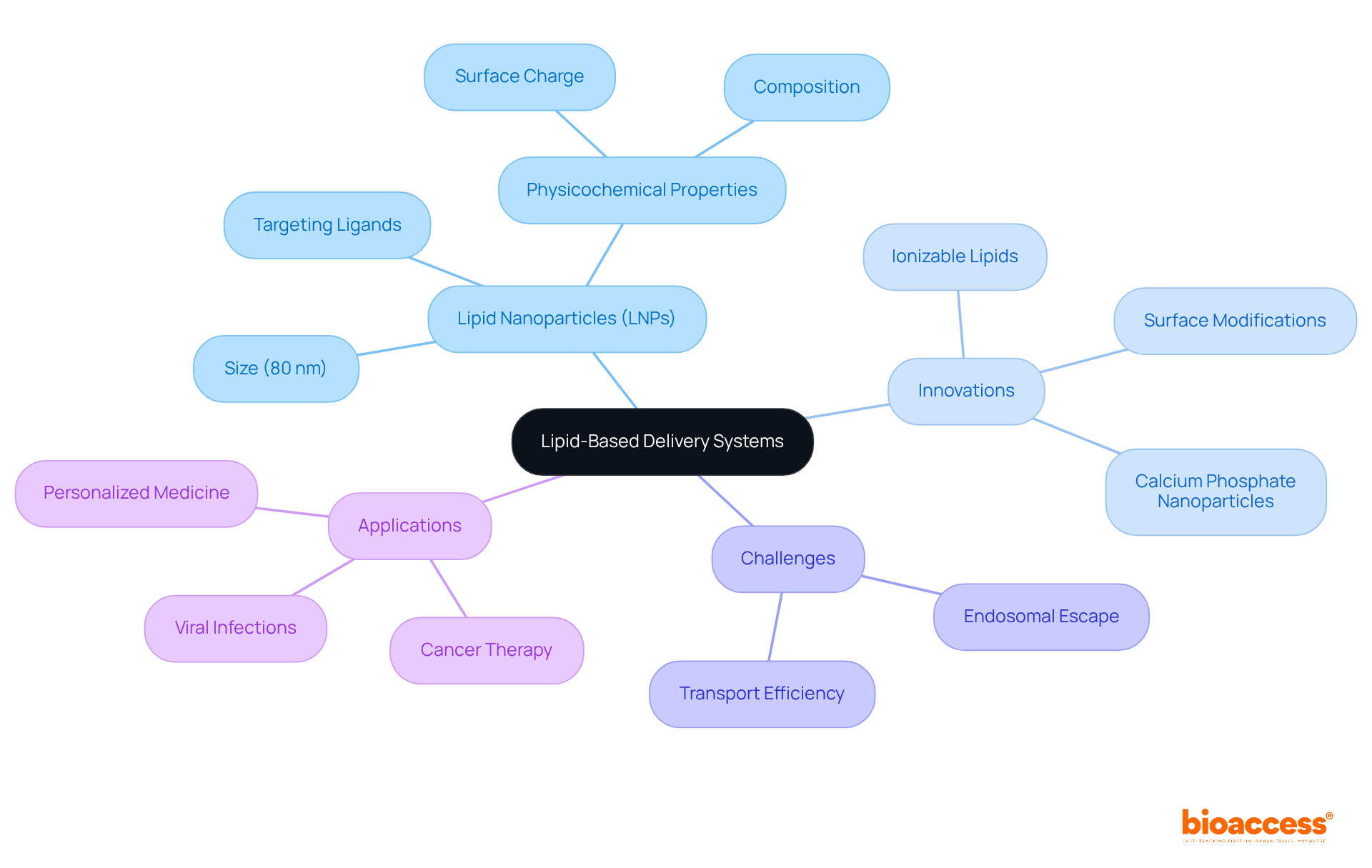
Lipid-based transport systems, especially lipid nanoparticles (LNPs), are pivotal in siRNA therapeutics. These advanced systems are designed as siRNA therapeutics that encapsulate small interfering RNA, protecting it from degradation while enhancing cellular uptake. Recent innovations in lipid formulations, such as ionizable lipids and helper lipids, have markedly increased siRNA transport efficiency, which is crucial for the advancement of siRNA therapeutics. Notably, studies reveal that LNPs approximately 80 nm in size exhibit maximum silencing activity, highlighting their potential in siRNA therapeutics.
Researchers underscore the necessity of optimizing the physicochemical properties of lipids to customize formulations for specific siRNA therapeutics applications. Advancements in surface modifications, particularly the incorporation of targeting ligands, have proven to enhance transport efficiency to tumor sites for siRNA therapeutics. Recent findings illustrate that lipid-coated calcium phosphate nanoparticles significantly improve cellular uptake in triple-negative breast cancer cells, showcasing the practical applications of these technologies.
Statistics reveal that merely around 2% of engineered RNA transport systems can successfully evade endosomes, highlighting persistent challenges in achieving high transport efficiency. Nevertheless, ongoing research into LNPs is paving the way for innovative solutions in siRNA therapeutics, with engineered LNPs showing enhanced treatment effects by shedding targeting moieties upon reaching their destination. This advancement not only refines the precision of RNA transport but also holds promise for overcoming biological hurdles in siRNA therapeutics.
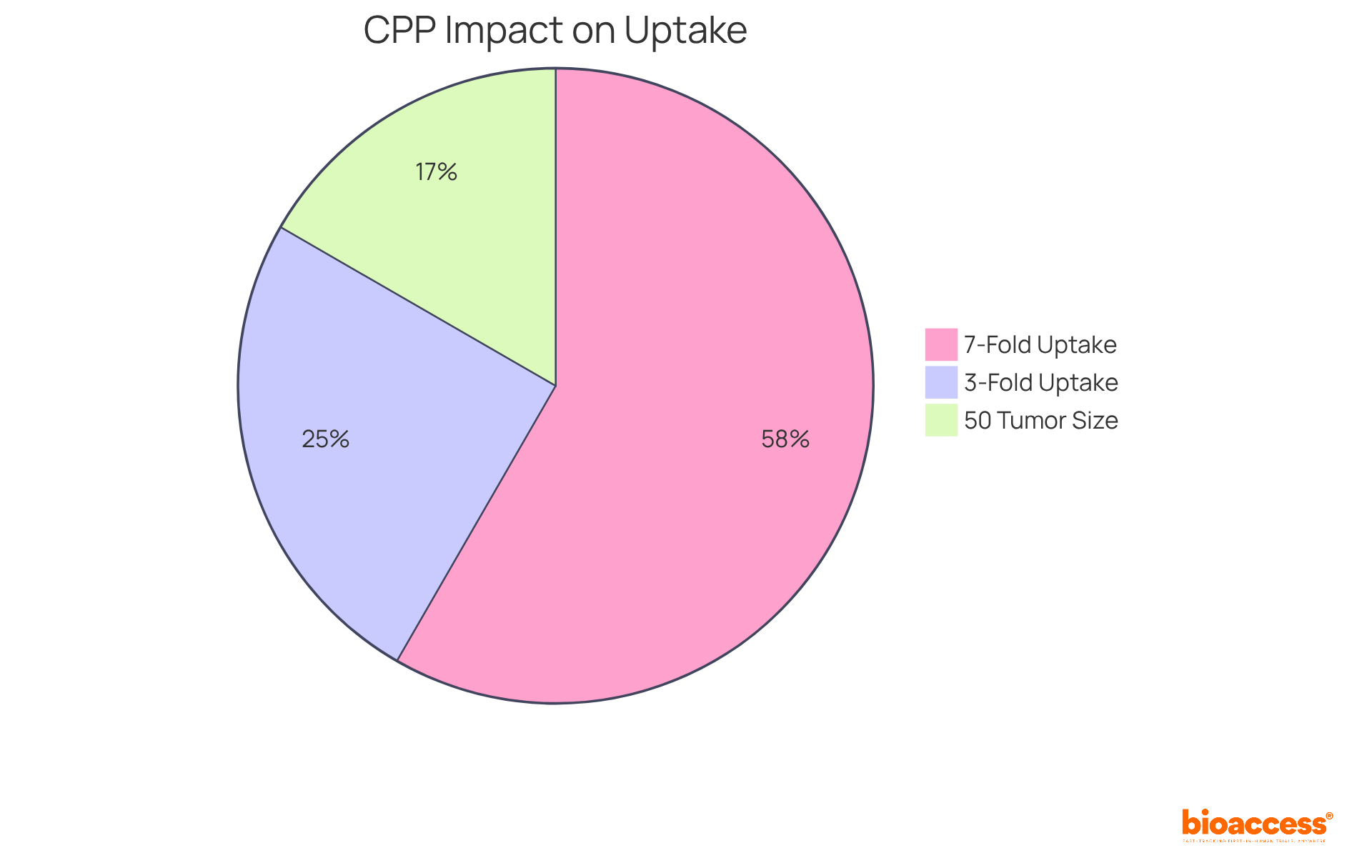
Incorporating cell-penetrating peptides (CPPs) into RNA interference transport systems significantly enhances cellular absorption and therapeutic effectiveness. By facilitating the movement of small interfering RNA across membrane barriers, CPPs enable more efficient delivery of therapeutics. Research indicates that the application of specific CPPs can boost in vitro uptake by as much as 7-fold relative to controls, showcasing their potential in overcoming biological barriers.
The investigation of various CPPs and their distinct modes of action is essential for improving siRNA therapeutics, especially in challenging tissue types. Notably, siRNA therapeutics that involve the combination of siRNA with CPPs have been associated with enhanced biodistribution and reduced off-target effects. For instance, studies demonstrate that nanopolymer micelles containing RACPP and PTX resulted in a 3-fold increase in cell uptake and a 50% reduction in tumor size.
However, several challenges must be addressed to fully realize the potential of CPPs in enhancing the effectiveness of nucleic acid-based therapies. These include considerations related to the route of administration, in vivo stability, and non-specific uptake. Addressing these challenges will be crucial in advancing the application of CPPs in clinical research.
The exploration of strategies to enhance siRNA therapeutics delivery and efficacy reveals the critical importance of overcoming both extracellular and intracellular challenges. By leveraging innovative approaches such as nucleotide modifications, formulation-based delivery systems, and advanced transport mechanisms, the field is poised to significantly improve the effectiveness of RNA interference treatments. These advancements not only pave the way for more efficient therapeutic applications but also highlight the necessity of strategic partnerships, such as those offered by bioaccess®, to expedite clinical research and regulatory navigation.
Key arguments presented emphasize the role of:
in optimizing siRNA delivery. Each strategy contributes uniquely to the overall efficacy of therapeutic interventions, showcasing the ongoing evolution in the design and implementation of siRNA treatments. The integration of cell-penetrating peptides further underscores the potential for enhanced cellular uptake, which is essential for maximizing therapeutic impact.
Reflecting on these insights, it becomes clear that the future of siRNA therapeutics hinges on continued innovation and collaboration across the industry. As research progresses and new methods emerge, stakeholders are encouraged to remain engaged and informed about the latest developments. By doing so, they can effectively contribute to the advancement of precision medicine, ultimately leading to improved outcomes for patients worldwide.
What is bioaccess® and what does it specialize in?
bioaccess® specializes in expediting clinical research for siRNA therapeutics by effectively navigating the regulatory landscapes of Latin America, the Balkans, and Australia.
How quickly can bioaccess® secure ethical approvals for clinical trials?
bioaccess® can secure ethical approvals in a remarkable 4-6 weeks.
How does the enrollment process at bioaccess® compare to traditional markets?
The enrollment processes at bioaccess® are 50% faster than those in traditional markets.
What services does bioaccess® provide for clinical trials?
bioaccess® offers comprehensive clinical trial management services, including feasibility studies, site selection, compliance reviews, and project management.
Why is regulatory speed important for clinical trial success?
Regulatory speed is crucial because it significantly impacts clinical trial success rates, allowing companies to maintain competitiveness in the evolving treatment landscape.
What challenges do siRNA therapeutics face in administration?
siRNA therapeutics face substantial extracellular challenges, such as evading degradation by nucleases and navigating biological barriers, as well as intracellular challenges, including effective absorption by target cells and escaping endosomal compartments.
What strategies are employed to enhance the delivery of siRNA therapeutics?
Strategies include the use of protective formulations and advanced delivery vehicles to enhance cellular uptake and facilitate endosomal escape.