


The primary objective of this article is to delineate strategies for effectively leveraging the 510(k) FDA database to secure success within the Medtech industry. It is crucial to understand the submission process, identify predicate devices, and utilize insights gleaned from previous applications. These critical strategies can significantly enhance the likelihood of obtaining timely FDA approvals and improving overall market entry success.
Navigating the intricate landscape of medical device regulation presents significant challenges for Medtech innovators, particularly regarding the pivotal 510(k) submission process. This comprehensive database not only serves as a gateway to market entry in the U.S. but also provides invaluable insights that can shape successful application strategies.
However, with nearly one-third of submissions facing rejection, it becomes essential to understand how to effectively leverage the 510(k) FDA database.
What strategies can Medtech companies employ to enhance their chances of success and streamline their path to approval?
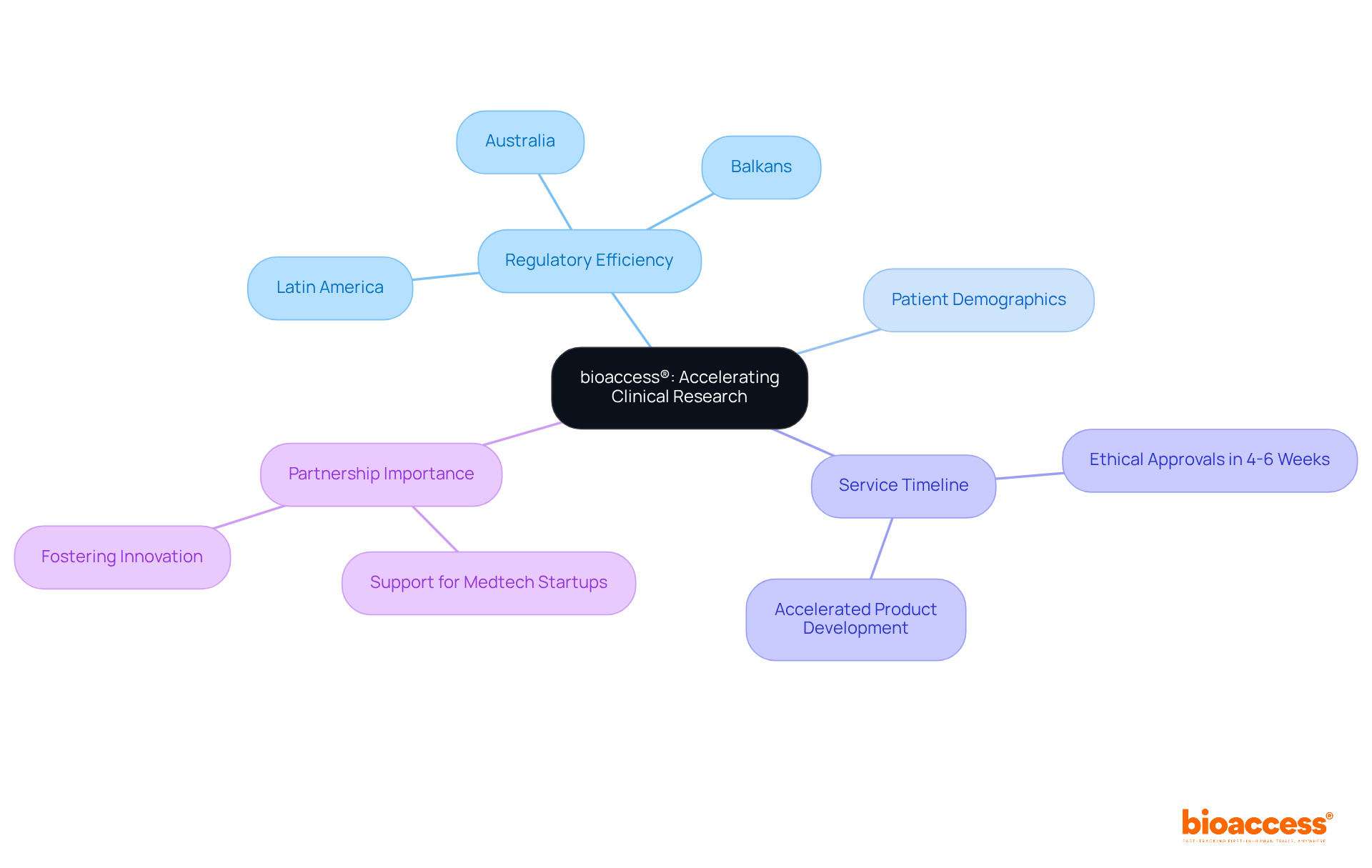
bioaccess® excels in delivering rapid clinical research services tailored for Medtech innovators. By leveraging the regulatory efficiency of Latin America, the diverse patient demographics in the Balkans, and the streamlined pathways in Australia, bioaccess® achieves ethical approvals in an impressive 4-6 weeks. This expedited process significantly accelerates product development timelines, allowing Medtech companies to enter the market more swiftly and improve patient outcomes.
With over 15 years of expertise in early-phase clinical studies, bioaccess® stands as a vital ally for Medtech startups navigating a competitive landscape. Industry leaders underscore that rapid clinical research services are critical for fostering innovation and meeting the evolving demands of healthcare.
As trends in 2025 suggest a heightened focus on agility and efficiency in clinical trials, successful partnerships in early-phase studies will be essential for Medtech companies striving to thrive in this dynamic environment.
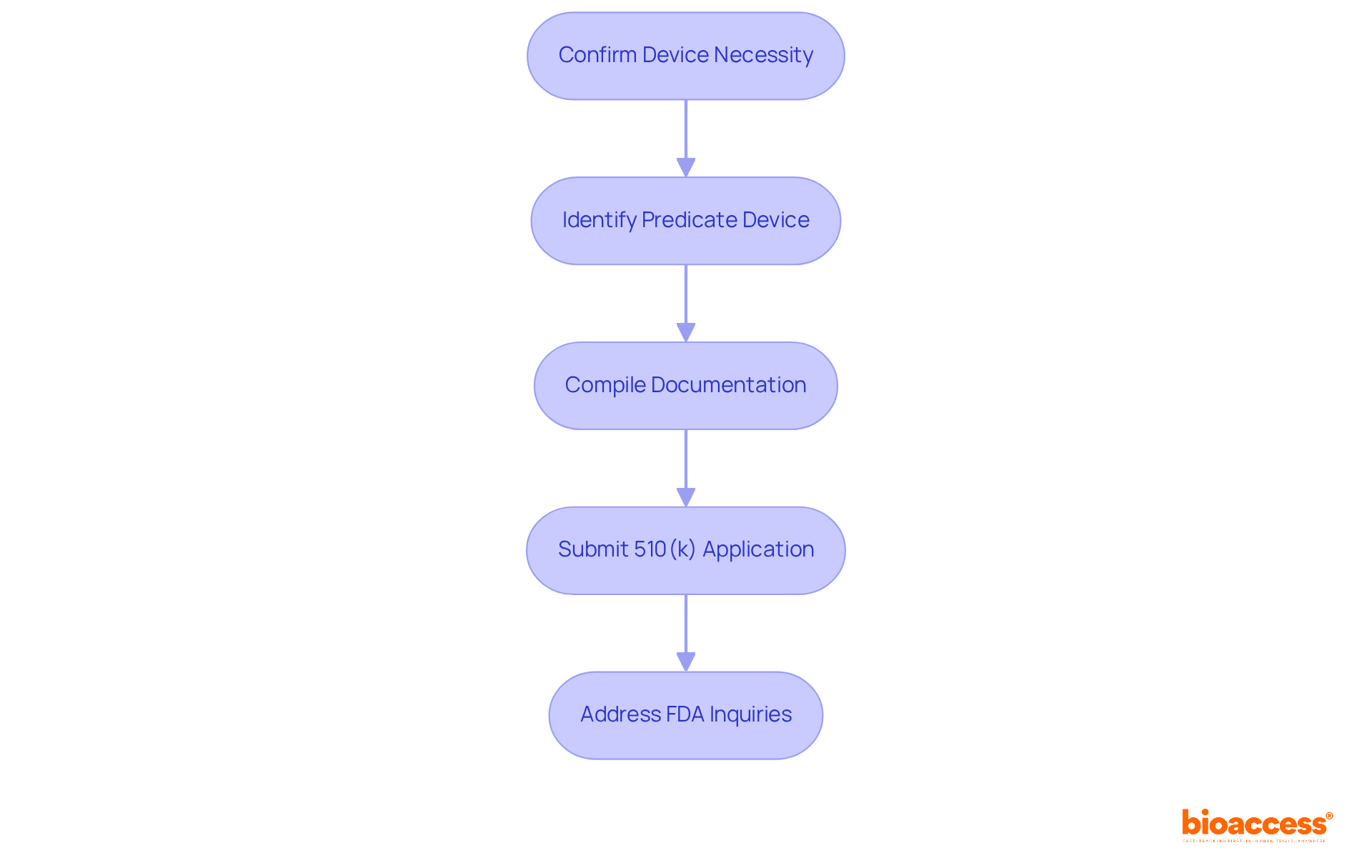
The application process for medical devices, detailed in the 510 k fda database, is crucial for bringing these products to market in the U.S. and encompasses several critical steps.
With 30% of entries in 2022 not accepted for initial evaluation, understanding these steps is vital for compliance and ensuring a smooth review process. Regulatory experts, including Ana Criado and Katherine Ruiz, emphasize that meticulous preparation is essential to avoid common pitfalls that can delay market entry. A right-first-time approach can significantly expedite market entry, reducing wasted time and resources. As Alex Pavlović states, "A right-first-time 510(k) approach gets you to market faster, eliminating wasted time and effort while generating revenue more quickly."
According to the 510 k fda database, the average approval time for a 510(k) application is 175 days, with a median of 85 days, underscoring the importance of comprehensive documentation and preparation. Additionally, potential costs associated with third-party testing should be considered, as they can impact the financial planning of Medtech companies navigating the 510(k) submission process.
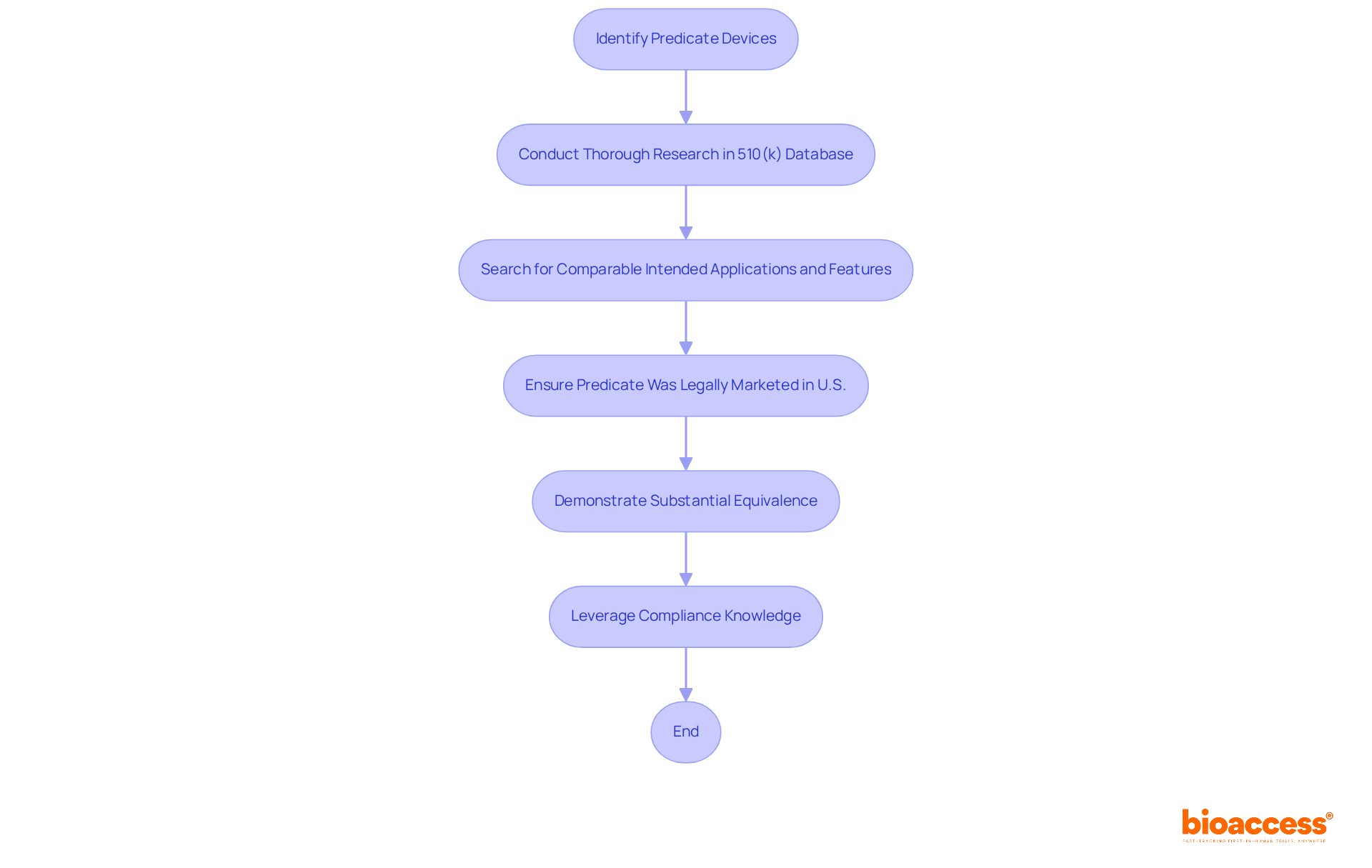
To identify an appropriate predicate product, manufacturers must conduct thorough research within the 510 k FDA database. It is crucial to search for items that possess comparable intended applications and technological features. The predicate apparatus must have been legally marketed in the U.S. prior to the submission of your product. This comparison is essential for demonstrating substantial equivalence, a key requirement for obtaining clearance from the 510 k FDA database. Experts emphasize the importance of leveraging extensive compliance knowledge to navigate this process effectively. Furthermore, comprehending both local and international regulatory environments is vital when identifying predicate instruments.
The 510 k FDA database application process presents several common challenges that can hinder approval. Key issues include:
To navigate these pitfalls effectively, it is crucial to ensure that all documentation is comprehensive, precise, and well-organized. Interacting with compliance advisors can offer beneficial perspectives and assistance, aiding in clarifying intricate requirements and simplifying the application process. For instance, companies that have successfully overcome these challenges often emphasize the importance of thorough pre-submission planning and the use of detailed checklists to ensure compliance with FDA expectations. By adopting these strategies, Medtech innovators can enhance their chances of a successful application in the 510 k FDA database.

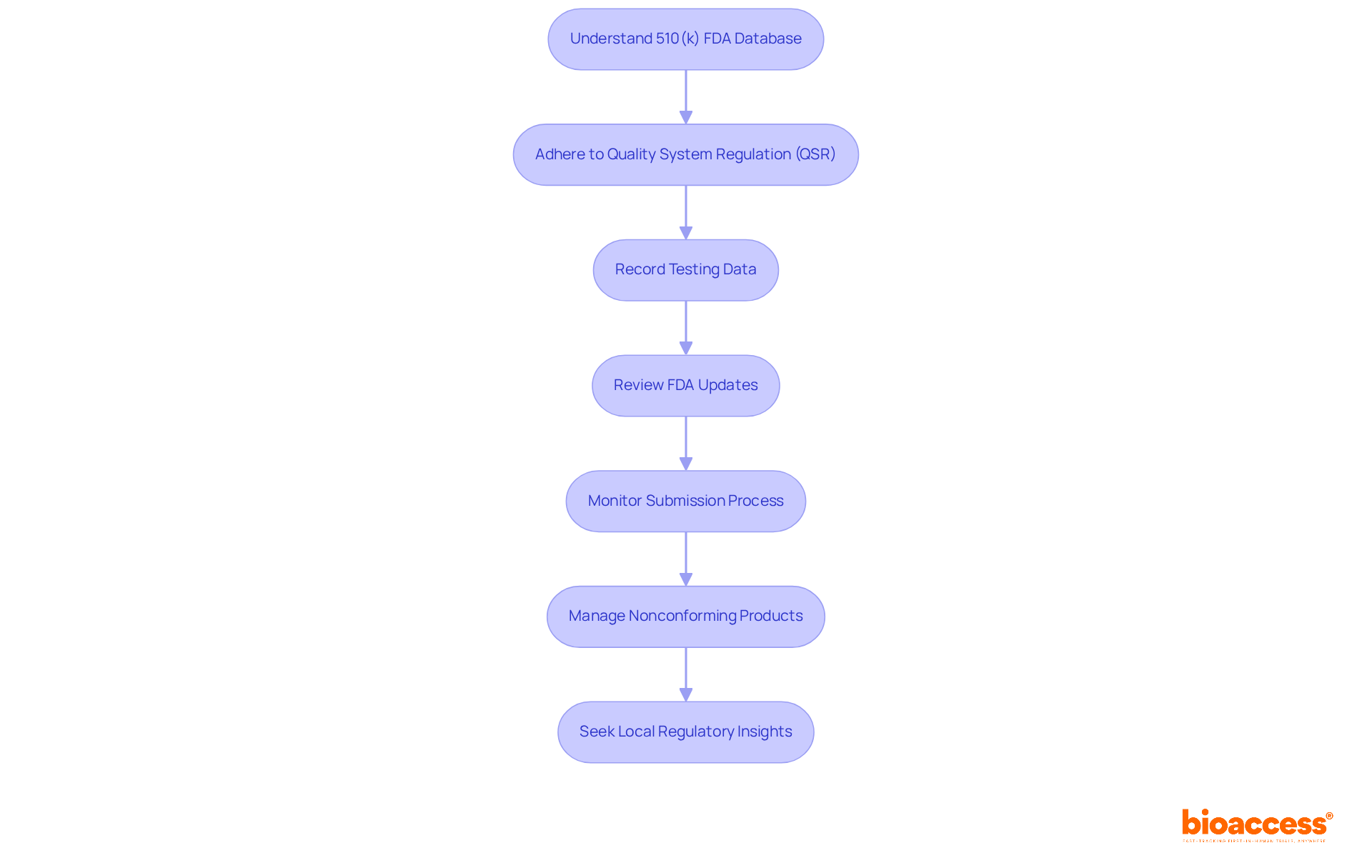
To ensure compliance with regulations during the 510(k) application process, manufacturers must possess a thorough understanding of the 510 k FDA database, including its guidelines and requirements. Adherence to the Quality System Regulation (QSR) is essential, as it mandates that all records related to the Quality Management System (QMS) be accurate, complete, and accessible. Comprehensive and meticulously recorded testing data is critical for a successful entry.
Regularly reviewing updates from the FDA is imperative, considering that approximately 30% of applications listed in the 510 k FDA database were placed on Refuse to Accept (RTA) hold in 2018 due to inconsistencies. Establishing a proactive approach for oversight monitoring can significantly enhance a company's ability to navigate the complexities of the submission process and secure timely approvals.
Furthermore, manufacturers must implement processes for identifying, documenting, and managing nonconforming products, which is a crucial aspect of compliance under Title 21 Part 820. Experts underscore the necessity of understanding local regulations, particularly in markets such as Colombia, where insights from seasoned regulatory consultants can offer invaluable guidance in effectively navigating these challenges.
When receiving feedback from the FDA, it is essential to analyze the comments and requests for additional information meticulously. Addressing any concerns raised by the FDA should be a top priority, as neglecting this can lead to delays or even denial of the application. Clear communication with the FDA is crucial; therefore, consider scheduling a follow-up meeting to clarify any points of confusion. Additionally, document all interactions to ensure a comprehensive understanding of the feedback provided.
The 510 k FDA database program is undergoing continuous enhancements aimed at improving both efficiency and safety. Medtech innovators, including experts like Ana Criado, Director of Regulatory Affairs and a professor in biomedical engineering, must stay abreast of new guidelines, such as the introduction of the eSTAR (electronic Submission Template and Resource) for applications. Grasping these changes is crucial for companies to adapt their submission strategies and ensure compliance with the latest requirements.
Regularly reviewing FDA updates and participating in industry discussions can provide valuable insights into upcoming modifications, particularly for those involved in oversight of medical instruments and in vitro diagnostics. Additionally, insights from Katherine Ruiz, a compliance specialist for medical devices and in vitro diagnostics in Colombia, can further deepen understanding of these evolving guidelines.
To foster effective communication with regulatory agencies, Medtech firms must prioritize transparency throughout the application process. Establishing clear lines of communication is essential, as it cultivates a collaborative relationship with the FDA. Engaging in pre-submission meetings can significantly enhance the likelihood of first-cycle approvals; studies indicate that products receiving such early engagement boast a 52% approval rate, compared to just 29% for those that do not. These meetings provide a crucial opportunity to clarify expectations and address potential concerns prior to formal submission, ultimately streamlining the review process.
Furthermore, maintaining well-organized and concise documentation is paramount. The 510(k) summary must encompass a description of the product's attributes alongside a comprehensive list of all product components, facilitating understanding and demonstrating professionalism and a commitment to compliance. Effective documentation should also feature detailed comparisons with predicate devices and evidence of substantial equivalence while adhering to accepted standards. Timely responses to any inquiries from the FDA reinforce a company's dedication to compliance with regulations, which is essential for successful filings in the 510 k fda database. As emphasized by industry specialists such as Ana Criado and Katherine Ruiz, who possess significant expertise in regulatory matters, comprehensive and transparent documentation is fundamental to effective regulatory filings, ensuring that all essential information is readily accessible and clearly articulated.
The 510 k FDA database is a crucial resource for Medtech innovators aiming to enhance their application strategies. By meticulously analyzing previously cleared devices, companies can extract valuable insights into effective practices, such as identifying common predicate devices and mastering documentation techniques. This 510 k FDA database empowers innovators to track approval trends, showing which data types have historically led to successful applications. Notably, around 85 percent of 510(k) applications receive a Substantially Equivalent decision, underscoring the importance of aligning with established benchmarks. By harnessing these insights, companies can refine their strategies, significantly boosting their chances of securing a successful 510(k) clearance. Industry leaders stress the necessity of leveraging these trends to inform strategic decisions, ensuring that submissions are compliant and competitive within the dynamic regulatory landscape.
Leveraging the 510(k) FDA database is imperative for Medtech innovators striving to navigate the complexities of regulatory submissions with efficiency. By comprehensively understanding the submission process, identifying predicate devices, and maintaining effective communication with regulatory bodies, companies can significantly enhance their prospects for successful product approvals. The insights garnered from the 510(k) database not only streamline applications but also cultivate a deeper understanding of market trends and compliance requirements.
Throughout this article, key strategies have been articulated, emphasizing the critical nature of meticulous documentation, proactive engagement with the FDA, and the necessity of thorough pre-submission planning. These elements are essential in overcoming common pitfalls that can impede market entry, such as incomplete submissions and misidentified predicate devices. Moreover, remaining informed about evolving regulations and leveraging expert guidance can further strengthen a company's position within the competitive Medtech landscape.
As the Medtech industry continues to transform, embracing these strategies will be vital for companies aiming to excel. Innovators are encouraged to actively engage with the 510(k) FDA database, refine their submission approaches, and prioritize compliance. By doing so, they not only enhance their chances of successful applications but also contribute to advancing healthcare solutions that ultimately improve patient outcomes.
What services does bioaccess® provide for Medtech innovators?
bioaccess® offers rapid clinical research services tailored for Medtech innovators, leveraging regulatory efficiency in Latin America, diverse patient demographics in the Balkans, and streamlined pathways in Australia to achieve ethical approvals in 4-6 weeks.
How does bioaccess® impact product development timelines for Medtech companies?
By providing expedited ethical approvals, bioaccess® significantly accelerates product development timelines, allowing Medtech companies to enter the market more swiftly and improve patient outcomes.
What experience does bioaccess® have in clinical studies?
bioaccess® has over 15 years of expertise in early-phase clinical studies, making it a vital ally for Medtech startups in a competitive landscape.
What is the significance of rapid clinical research services in the Medtech industry?
Rapid clinical research services are critical for fostering innovation and meeting the evolving demands of healthcare, especially as trends in 2025 indicate a focus on agility and efficiency in clinical trials.
What are the key steps in the 510(k) submission process for medical devices?
The key steps include confirming if a submission is necessary, identifying a suitable predicate device, compiling comprehensive documentation, and submitting the 510(k) application to the FDA.
Why is it important to understand the 510(k) submission process?
Understanding the process is vital for compliance and ensuring a smooth review, as 30% of entries in 2022 were not accepted for initial evaluation. Proper preparation can avoid delays and expedite market entry.
What is the average approval time for a 510(k) application?
The average approval time for a 510(k) application is 175 days, with a median of 85 days.
How can manufacturers identify appropriate predicate devices for 510(k) submissions?
Manufacturers can identify predicate devices by conducting thorough research within the 510(k) FDA database, looking for items with comparable intended applications and technological features that have been legally marketed in the U.S. prior to submission.
What is the importance of demonstrating substantial equivalence in 510(k) submissions?
Demonstrating substantial equivalence is a key requirement for obtaining clearance from the 510(k) FDA database, making it essential for manufacturers to identify appropriate predicate devices effectively.
What should Medtech companies consider regarding costs in the 510(k) submission process?
Medtech companies should consider potential costs associated with third-party testing, as these can impact their financial planning while navigating the 510(k) submission process.