


The article outlines seven ways in which bioaccess® accelerates medical research challenges, emphasizing its capability to expedite clinical trials and enhance patient outcomes. This is achieved through strategic advantages in regulatory processes, diverse patient recruitment, and cost-effective research solutions. By leveraging its unique position in Latin America, bioaccess® facilitates faster ethical approvals and participant enrollment. Furthermore, it promotes collaboration between Medtech and Biopharma, effectively streamlining the path from innovation to market. As a result, access to advanced medical treatments is significantly improved.
The rapid evolution of the medical research landscape presents both opportunities and challenges for innovators in Medtech, Biopharma, and Radiopharma. As the demand for efficient clinical trials surges, organizations like bioaccess® are stepping up to transform how research is conducted, particularly in regions such as Latin America and the Balkans. This article delves into seven impactful strategies employed by bioaccess® that not only streamline the research process but also enhance patient care and access to innovative treatments.
How can these advancements redefine the future of medical research and ultimately improve health outcomes for diverse populations?
The company is committed to empowering innovators in Medtech, Biopharma, and Radiopharma by delivering swift and effective research services. By concentrating on early-phase studies, the organization harnesses its extensive experience and regional advantages to expedite ethical approvals and participant enrollment. This strategy not only reduces the timeline from innovation to market but also significantly boosts the likelihood of successful outcomes. For instance, research studies in Latin America have demonstrated that bioaccess® can secure ethical approvals in just 4-6 weeks, facilitating enrollment rates that are 50% faster than those in conventional markets.
The impact of rapid medical research is profound, enabling quicker iterations and modifications of health technologies, ultimately enhancing cured medical care for individuals. With the medical trials market projected to reach approximately USD 886.5 billion by 2032, the demand for efficient and effective research solutions has never been more critical. By leveraging its unique position in Latin America, the company not only accelerates the research process but also contributes to the broader advancements in Medtech and Biopharma, ensuring that innovative treatments reach patients in need without unnecessary delays.
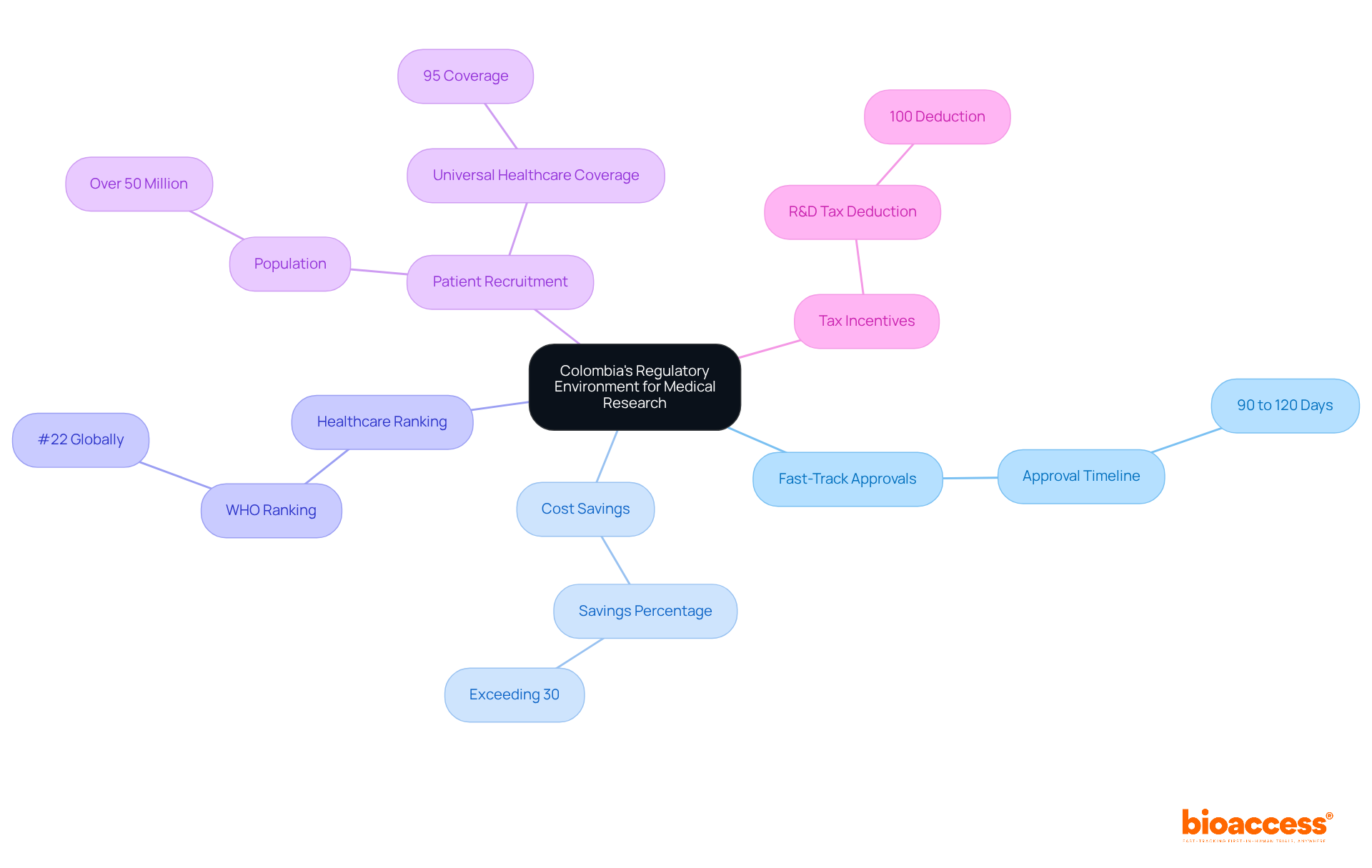
Latin America, particularly Colombia, presents a distinctive regulatory environment that facilitates fast-track approvals, enabling bioaccess® to secure ethical approvals within a mere 90 to 120 days. This expedited regulatory process not only accelerates the initiation of research studies but also enhances the overall effectiveness of research activities.
With cost savings exceeding 30% compared to trials conducted in North America or Western Europe, alongside a healthcare system ranked #22 globally by the World Health Organization, Colombia emerges as an appealing option for innovators aiming to swiftly bring their products to market.
Moreover, the nation's robust patient recruitment capabilities, supported by a population surpassing 50 million and universal healthcare coverage for 95% of its citizens, further solidify its position as a premier destination for medical research.
Additionally, investments in science, technology, and innovation projects in Colombia benefit from substantial R&D tax incentives, including a 100% tax deduction and various future tax credits. Hospitals in Colombia must also undergo a stringent ICH/GCP certification process to conduct research involving human subjects, thereby ensuring high-quality standards.
The Balkans present a unique and diverse demographic landscape, essential for conducting comprehensive research trials. By capitalizing on this rich variety of patient backgrounds, bioaccess® significantly bolsters the validity and applicability of its research findings, ensuring that results resonate with a broader spectrum of patient experiences. This diversity not only elevates the quality of the data collected but also cultivates greater trust in the outcomes of medical studies.
Research studies involving individuals from varied ethnic and socioeconomic backgrounds have demonstrated enhanced treatment responses and more reliable data, ultimately leading to improved healthcare solutions. The commitment to inclusivity in cured medical research is paramount, as it facilitates a deeper understanding of how different populations respond to treatments, thereby addressing health disparities and advancing equitable healthcare progress.

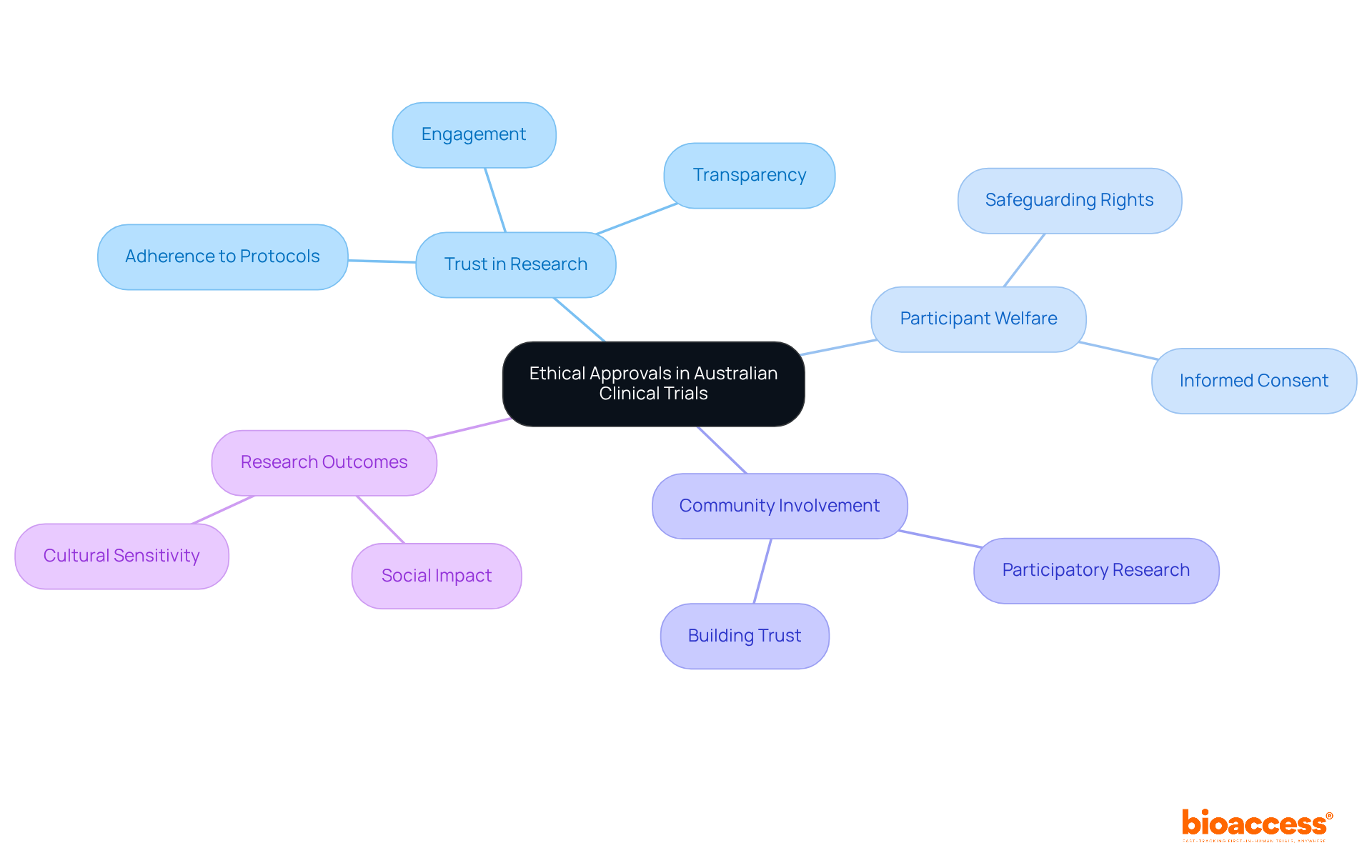
In Australia, rigorous ethical standards are upheld, ensuring that all research trials are conducted with the utmost integrity. This unwavering commitment to ethical practices not only safeguards participant welfare but also cultivates trust among stakeholders, including regulatory bodies and the public. By prioritizing ethical approvals, typically secured within 4 to 6 weeks, bioaccess® significantly enhances the credibility of its research initiatives.
Recent data underscores that trust in medical research is paramount, as participants express a strong preference for transparency and ethical conduct. For instance, studies indicate that when ethical standards are upheld, participants are more likely to engage proactively and adhere to trial protocols.
Furthermore, successful examples of trust-building in medical research highlight the effectiveness of community involvement and clear communication strategies. These practices align with the ethical principles of respect and autonomy, reinforcing the collective responsibility of researchers to honor participant contributions. Ultimately, this leads to more robust and socially impactful research outcomes.
bioaccess® excels in conducting early-phase studies, particularly first-in-human (FIH) and early-feasibility studies (EFS), which are essential for evaluating the safety and efficacy of emerging medical technologies. These studies serve as the foundation for subsequent medical experiments, facilitating the transition from innovative ideas to practical healthcare solutions.
The importance of FIH studies is highlighted by the fact that approximately 70% of investigational agents entering Phase I progress to Phase II, underscoring their critical role in the drug development pipeline. As of 2023, the global landscape reveals over 20,109 drugs in the R&D pipeline, reflecting a robust commitment to innovation. Furthermore, the average completion rate for Phase I trials stands at an impressive 91.4%, indicating a high level of success in these early stages.
By emphasizing early-stage research, the organization not only accelerates the creation of innovative therapies but also enhances care and outcomes, establishing itself as a leader in the research field. Significantly, this technology enables treatment-naive cardiology or neurology groups to enroll 50% faster than Western locations, achieving substantial cost reductions of $25K per individual with FDA-ready data—no rework, no delays.
This competitive advantage is further illustrated by collaborations with companies such as Avantec Vascular, which selected a specific technology for its first-in-human clinical study in Latin America, showcasing the organization's capability to navigate complex regulatory environments and facilitate patient recruitment.
As indicated in a recent survey, "56 percent of sites claim trials are more complicated than merely three years ago," emphasizing the importance of organizations like bioaccess in addressing these challenges.

The company provides robust market access services that empower clients to harness the immense potential of Latin America's healthcare market, which is characterized by a rapidly growing population and increasing healthcare expenditures. With over 20 years of experience in Medtech, our team adeptly navigates the complexities of market entry, enabling innovators to effectively commercialize their products and ensuring that groundbreaking medical technologies reach those who need them most. This strategic approach not only enhances the visibility of new products but also stimulates growth within the healthcare sector.
As the Latin American pharmaceutical market is projected to expand significantly, driven by rising incomes and an aging population, the demand for innovative healthcare solutions is at an unprecedented high. Organizations that leverage the expertise of this firm can position themselves advantageously in this dynamic environment, seizing opportunities for improved health outcomes while contributing to job creation and economic development in the region.
To maximize these benefits, collaborating with this organization can be a crucial step in navigating the complexities of clinical trials and market access.

This initiative promotes cooperation between Medtech and Biopharma firms, recognizing that such alliances are essential for driving innovation and improving health outcomes. By linking these sectors, the platform facilitates the sharing of knowledge, resources, and expertise, which is crucial for creating more effective medical solutions. This collaborative framework not only streamlines the research process but also expedites the introduction of groundbreaking therapies to patients.
For example, Dushyanth Surakanti, Founder and CEO of Sparta Biomedical, shared his positive experience with bioaccess® during its initial human study in Colombia, showcasing the effectiveness of such collaborations. Furthermore, bioaccess™ has partnered with Caribbean Health Group to establish Barranquilla as a prominent location for research studies in Latin America, a decision endorsed by Colombia's Minister of Health. Additionally, bioaccess™ collaborates with IDx Technologies for AI-based disease detection, enhancing diagnostic capabilities.
These partnerships are further exemplified by collaborations with GlobalCare Clinical Trials, which has achieved over a 50% reduction in recruitment time and 95% retention rates in clinical trials. As healthcare evolves, the synergy between Medtech and Biopharma will play a pivotal role in shaping a more responsive and effective healthcare landscape.

The company is resolutely committed to providing affordable research solutions that empower Medtech, Biopharma, and Radiopharma innovators to launch their products to market without encountering substantial financial obstacles. By optimizing resources and streamlining processes, bioaccess® enhances the availability of innovative medical technologies for individuals in need. This dedication to affordability not only benefits clients but also significantly impacts the healthcare landscape by increasing access to advanced treatments.
With fewer than 5% of adult cancer patients participating in research studies, the demand for cost-effective innovations is paramount. For instance, leveraging technology and flexible study designs can substantially reduce expenses while maintaining quality. Furthermore, initiatives aimed at enhancing transparency in participant payments and addressing financial toxicity are crucial for improving access to clinical trials.
By focusing on these strategies, this approach is instrumental in ensuring that innovative medical advancements reach those who need them most.

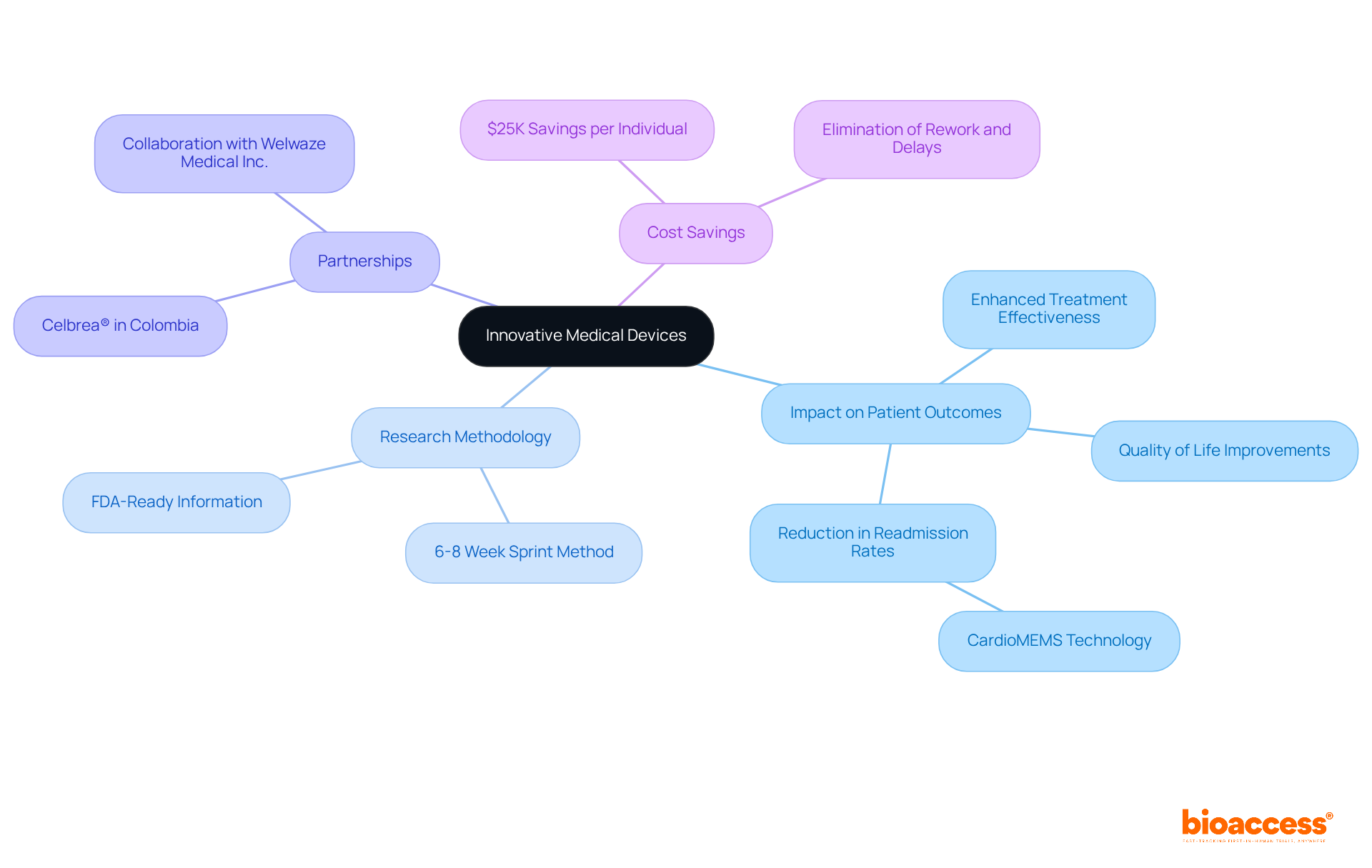
Cutting-edge medical devices are revolutionizing outcomes for individuals, with technology playing a crucial role in their development. By facilitating early-stage research through a streamlined 6-8 week sprint method, the platform aids in validating new technologies that can significantly enhance healthcare. This innovative approach enables treatment-naive cardiology or neurology groups to register 50% faster than their Western counterparts, accelerating research studies while achieving $25K savings per individual through FDA-ready information—effectively eliminating rework and delays. Such innovations not only improve treatment effectiveness but also elevate the quality of life for individuals, underscoring the organization's vital contributions to the healthcare sector.
The company has partnered with Welwaze Medical Inc. to support the launch of the Celbrea® medical device in Colombia, reinforcing its position as a strategic ally for cost-effective early-phase clinical trials in Medtech and Biopharma.
In a constantly evolving healthcare landscape, the company is steadfast in its commitment to fostering continuous advancements in care innovations. By meticulously tracking emerging technologies and trends—such as the anticipated growth of the AI healthcare market to $208.2 billion by 2030 and the expected expansion of the telehealth market to $590.9 billion by 2032—bioaccess® strategically positions itself to champion the next wave of medical breakthroughs. This proactive stance not only enhances the efficacy of medical research but also significantly improves outcomes for individuals.
For instance, the integration of big data analytics, projected to reach $794.08 billion by 2030, facilitates more tailored treatment strategies, ultimately benefiting both patients and healthcare providers. As bioaccess® navigates these advancements, it remains at the forefront of transforming clinical research, ensuring that innovations yield tangible benefits for patients.

bioaccess® stands at the forefront of transforming medical research, delivering innovative solutions that effectively tackle key challenges in the clinical trial landscape. By leveraging its unique capabilities in regions such as Latin America and the Balkans, the company accelerates the research process while enhancing the quality and accessibility of medical innovations. This strategic approach ensures that groundbreaking treatments reach patients more swiftly, ultimately improving healthcare outcomes.
Throughout this article, several critical aspects of bioaccess®'s services have been highlighted. The organization excels in:
Moreover, its unwavering commitment to ethical standards and cost-effective research solutions positions bioaccess® as a reliable partner in the healthcare sector, facilitating collaborations between Medtech and Biopharma that drive innovation.
As the demand for efficient medical research continues to grow, it is essential for stakeholders to recognize the significance of organizations like bioaccess®. By embracing advancements in clinical research and fostering collaboration, the healthcare industry can ensure that innovative solutions are not only developed but also made accessible to those who need them most. Engaging with bioaccess® represents a pivotal step in navigating the complexities of clinical trials, ultimately leading to a healthier future for all.
What is bioaccess® and what services does it provide?
bioaccess® is a company that empowers innovators in Medtech, Biopharma, and Radiopharma by delivering swift and effective research services, focusing on early-phase studies to expedite ethical approvals and participant enrollment.
How does bioaccess® impact the timeline from innovation to market?
By concentrating on early-phase studies and leveraging its extensive experience, bioaccess® reduces the timeline from innovation to market and significantly boosts the likelihood of successful outcomes.
What are the advantages of conducting research studies in Latin America through bioaccess®?
Research studies in Latin America can secure ethical approvals in just 4-6 weeks and facilitate enrollment rates that are 50% faster than those in conventional markets, enabling quicker iterations of health technologies.
What is the projected market size for medical trials by 2032?
The medical trials market is projected to reach approximately USD 886.5 billion by 2032.
What regulatory advantages does Colombia offer for clinical research?
Colombia allows bioaccess® to secure ethical approvals within 90 to 120 days, which accelerates the initiation of research studies and enhances overall research effectiveness.
How does the cost of conducting trials in Colombia compare to North America or Western Europe?
Trials conducted in Colombia can result in cost savings exceeding 30% compared to those conducted in North America or Western Europe.
What are the key features of Colombia's healthcare system that support medical research?
Colombia has a healthcare system ranked #22 globally by the World Health Organization, a population exceeding 50 million with universal healthcare coverage for 95% of its citizens, and robust patient recruitment capabilities.
What incentives are available for R&D investments in Colombia?
Investments in science, technology, and innovation projects in Colombia benefit from substantial R&D tax incentives, including a 100% tax deduction and various future tax credits.
What standards must hospitals in Colombia meet to conduct research involving human subjects?
Hospitals in Colombia must undergo a stringent ICH/GCP certification process to ensure high-quality standards in research involving human subjects.
How does bioaccess® utilize the diverse patient pools in the Balkans for clinical trials?
The diverse demographic landscape in the Balkans enhances the validity and applicability of research findings, leading to improved healthcare solutions and addressing health disparities through a deeper understanding of treatment responses across different populations.