


CDM Pharma significantly enhances drug development efficiency through rapid regulatory approvals, effective cost management, and advanced technological integration. This article underscores how Contract Development and Manufacturing Organizations (CDMOs) like bioaccess® achieve ethical approvals in a mere 4-6 weeks, while simultaneously cutting patient enrollment times by 50%. Additionally, they provide cost-effective solutions that empower startups and small biotech firms to optimize their budgets, thereby accelerating growth.
In the evolving Medtech landscape, the role of organizations like bioaccess® is crucial in addressing the pressing challenges faced by the industry. Their ability to streamline processes not only fosters innovation but also ensures that new therapies reach patients more swiftly. The collaboration between CDMOs and biotech firms is essential for navigating the complexities of clinical research, ultimately driving advancements in healthcare.
In summary, the significance of collaboration within the Medtech sector cannot be overstated. As CDM Pharma continues to lead the charge in enhancing drug development efficiency, stakeholders are encouraged to consider how they can leverage these partnerships to overcome their own challenges in clinical research.
The pharmaceutical landscape is in a constant state of evolution, driven by the urgent need for faster, more efficient drug development processes. CDM Pharma emerges as a pivotal player in this transformation, offering innovative solutions that not only streamline production but also optimize costs and ensure regulatory compliance. As the industry faces increasing pressure to deliver effective therapies swiftly, one must consider: how can Contract Development and Manufacturing Organizations (CDMOs) leverage their capabilities to enhance drug development efficiency and maintain a competitive edge in a rapidly changing market?
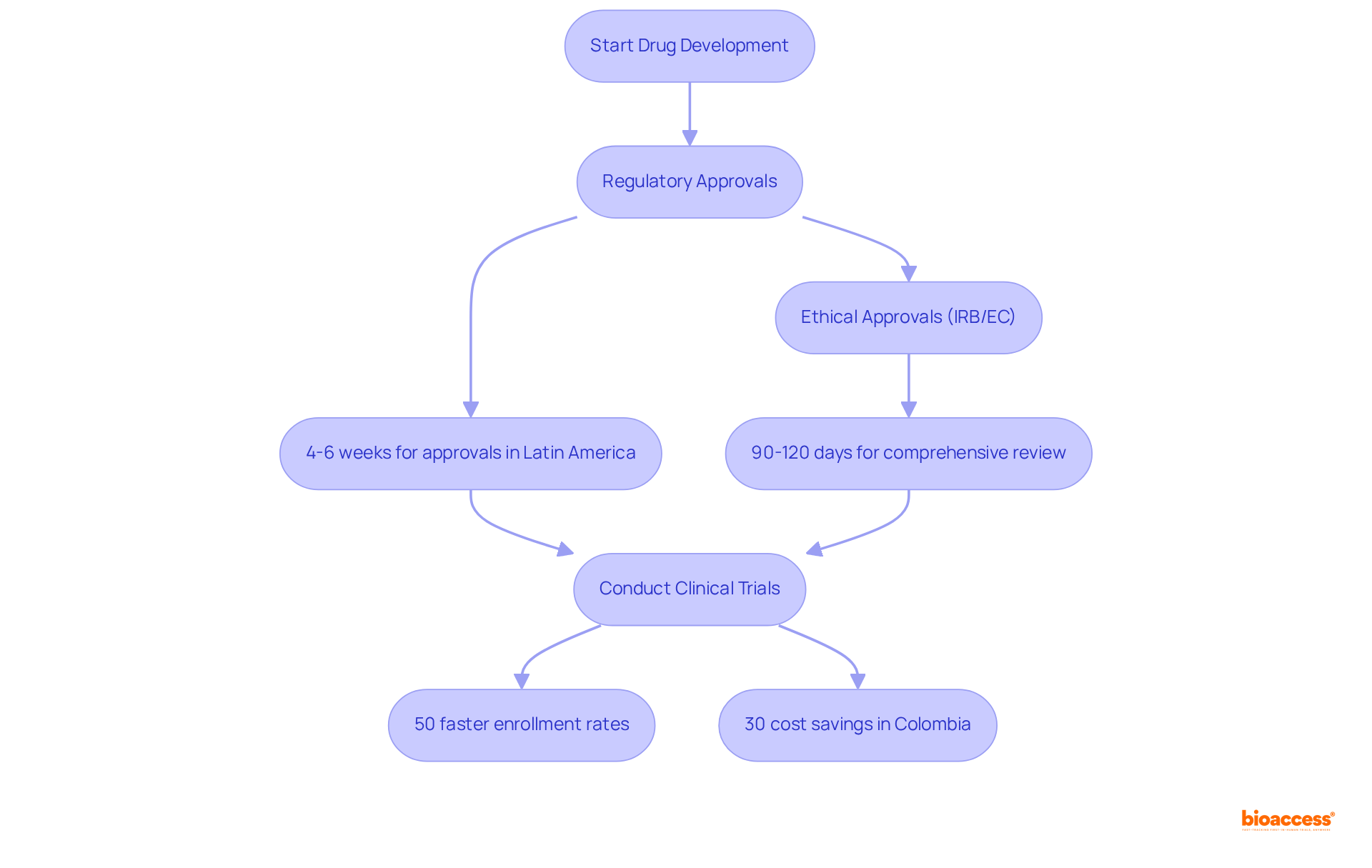
bioaccess® capitalizes on the regulatory speed of Latin America, the diverse patient populations in the Balkans, and Australia's efficient pathways to secure ethical approvals in an impressive 4-6 weeks. This pioneering clinical agility empowers Medtech, Biopharma, and Radiopharma innovators to significantly expedite their therapeutic development processes.
With enrollment rates that are 50% faster than traditional markets, bioaccess® facilitates quicker market access, enabling companies to deliver their innovative products to patients sooner. Furthermore, conducting trials in Colombia yields cost savings exceeding 30% compared to North America, with a comprehensive IRB/EC and MoH (INVIMA) review completed in just 90-120 days.
This strategic approach not only enhances the efficiency of clinical trials but also positions bioaccess® as a frontrunner in the rapidly evolving landscape of medical technology and pharmaceuticals.
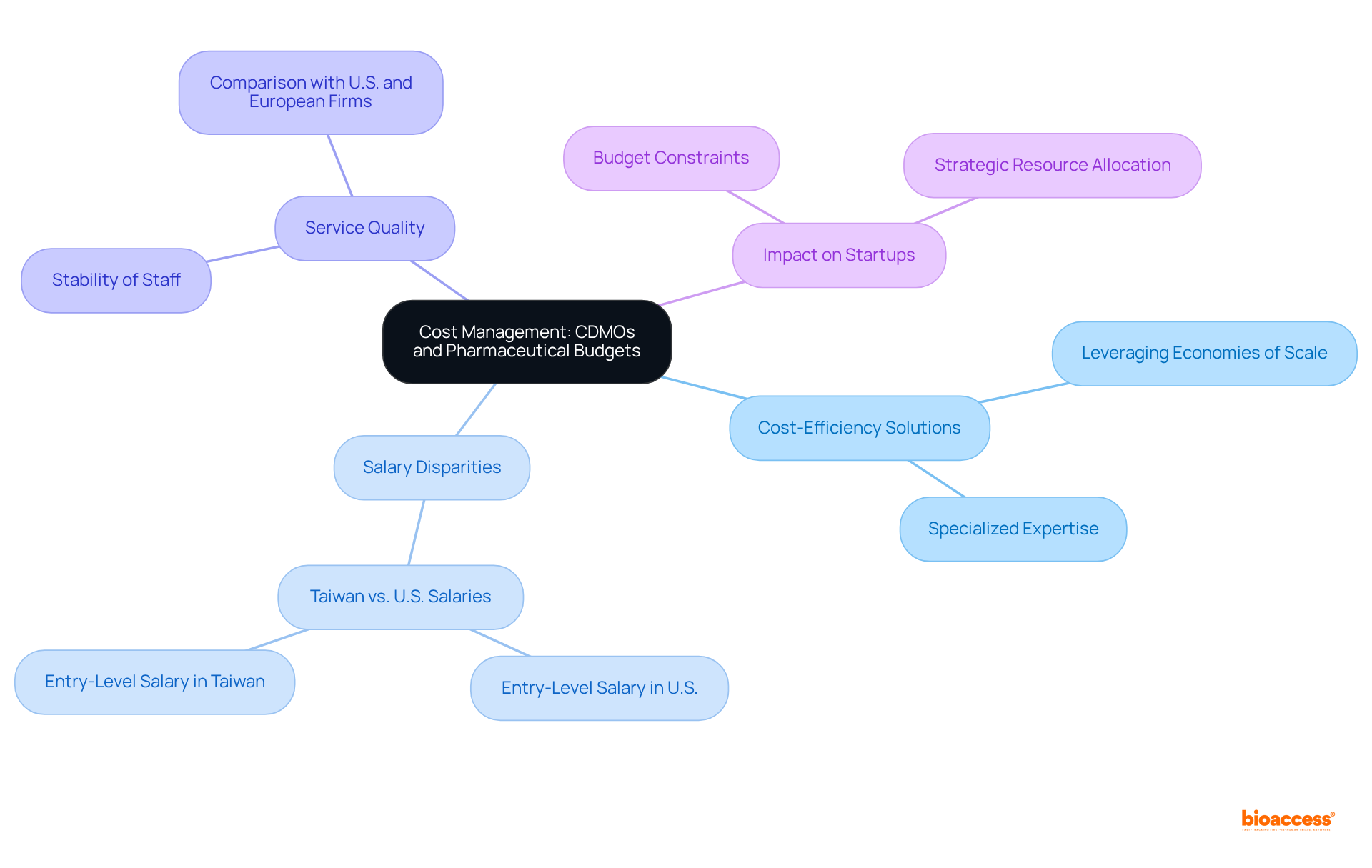
CDM pharma organizations are pivotal in optimizing pharmaceutical budgets by delivering cost-efficient solutions tailored to the unique needs of drug developers. By leveraging economies of scale and specialized expertise, these organizations can significantly reduce operational expenses, thereby enabling companies to allocate their resources more strategically. This optimization proves particularly advantageous for startups and small biotech firms that may operate with constrained budgets.
For instance, professionals in Taiwan, where many contract development and manufacturing organizations are based, often commence their careers at salaries that are substantially lower than their U.S. counterparts, typically earning half or one-third of the standard $90,000 entry-level salary. This cost disparity empowers contract development and manufacturing organizations, such as cdm pharma, to offer high-quality services at competitive rates, making them an attractive option for companies seeking to enhance their budget efficiency.
Furthermore, insights from industry leaders, including Jin-an Jiao, highlight concerns about the legitimacy of lower bids from contract manufacturing organizations, pointing to significant price discrepancies for similar work. Additionally, the stability of staff at contract development and manufacturing organizations in Taiwan contributes to improved service quality, contrasting with the high turnover rates seen in U.S. and European firms. Collectively, these factors enhance the effectiveness of medication development processes.
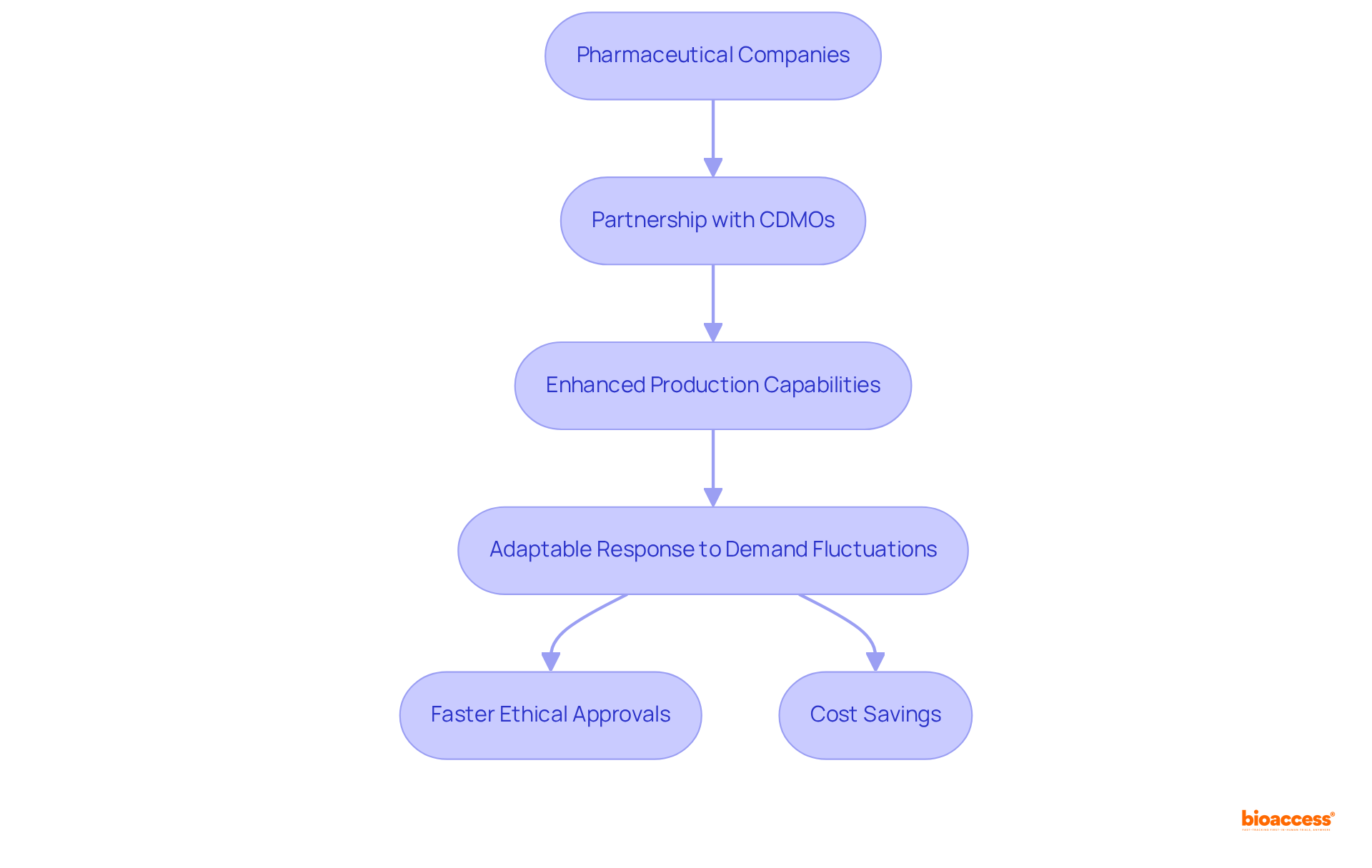
CDM Pharma plays a pivotal role in addressing the capacity constraints that pharmaceutical companies frequently encounter. They provide the essential infrastructure and specialized expertise necessary for efficient production scaling, empowering drug developers to meet increasing demand without compromising quality.
By forming partnerships with a CDMO, companies can swiftly enhance their production capabilities, allowing for a more adaptable response to fluctuations in demand. For instance, bioaccess® secures ethical approvals in just 4-6 weeks and achieves patient enrollment 50% faster than traditional markets, resulting in substantial savings of $25K per patient with FDA-ready data—no rework, no delays. This underscores the effectiveness of contract development and manufacturing organizations in streamlining clinical processes.
Moreover, CDM Pharma has successfully addressed specific capacity limitations in drug development, particularly in optimizing processes for biologics and mRNA-based products, which require advanced manufacturing capabilities. As the demand for complex therapeutics continues to rise, CDM Pharma companies are becoming indispensable partners in ensuring that pharmaceutical companies can scale their operations efficiently and effectively.
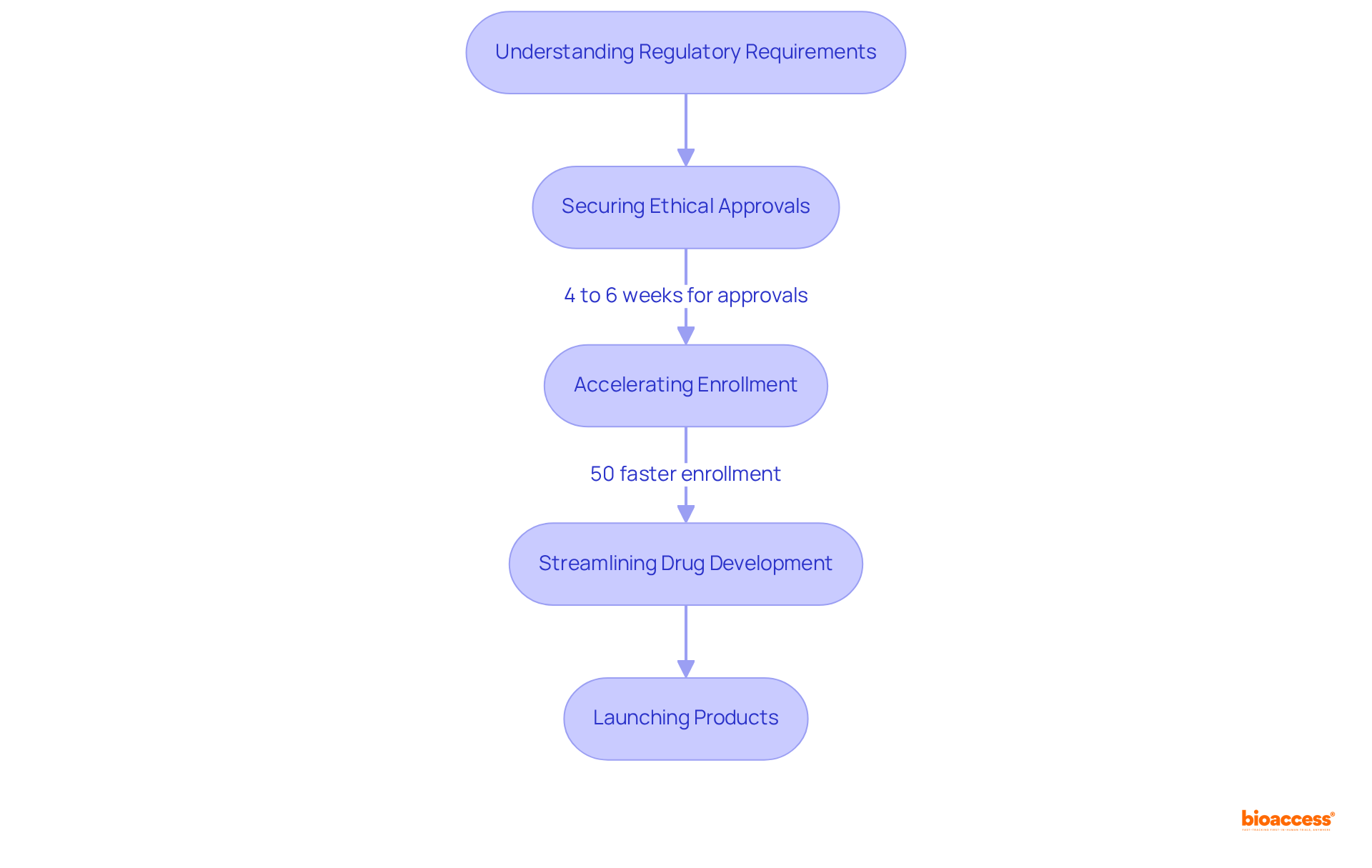
Navigating the complexities of regulatory compliance presents significant challenges for drug developers. CDM pharma organizations provide essential expertise in understanding and adhering to regulatory requirements, ensuring that all studies are conducted in strict compliance with established guidelines. This expertise not only mitigates risks but also accelerates the approval process, enabling companies to launch their products more swiftly.
For instance, bioaccess® secures ethical approvals in just 4 to 6 weeks, achieving enrollment 50% faster than conventional sectors. Such efficiency is crucial in a landscape where the average cost of bringing a new medication to market can exceed $2.6 billion and take more than ten years.
Regulatory experts assert that leveraging CDM Pharma capabilities can significantly streamline the drug development process, allowing innovators to focus on their core competencies while navigating the intricate regulatory landscape. Successful collaborations, such as the partnership between Samsung Biologics and Pfizer, illustrate how contract development and manufacturing organizations can enhance production capabilities and compliance, ultimately improving access to essential treatments.
By integrating regulatory knowledge with operational strategy, CDM Pharma plays a pivotal role in minimizing compliance obstacles and expediting the route to market for biopharmaceutical firms.
Contract manufacturing organizations like CDM Pharma are leading the charge in integrating technology and innovation into pharmaceutical creation processes. By leveraging advanced technologies such as data analytics, automation, and digital platforms, these organizations streamline operations, enhance data accuracy, and significantly improve overall efficiency. For instance, the utilization of AI and machine learning facilitates high-throughput screening and predictive modeling, which not only accelerates drug formulation but also mitigates costs and risks associated with traditional methods. In 2023, the Clinical Trial segment alone accounted for over 47.2% of the global contract development and manufacturing organization market, underscoring the increasing reliance on these entities for effective advancement.
Furthermore, collaborations between pharmaceutical firms and contract development manufacturing organizations are becoming increasingly essential. These partnerships allow pharmaceutical creators to concentrate on their core strengths while benefiting from advanced solutions that expedite the medication creation timeline. For example, bioaccess® provides comprehensive service capabilities, including:
This enables treatment-naive cardiology or neurology cohorts to enroll 50% faster than Western sites, achieving significant savings of $25K per patient with FDA-ready data—no rework, no delays. As the demand for groundbreaking treatments escalates, organizations like CDM Pharma are poised to enhance efficiency and effectiveness in pharmaceutical development, facilitating quicker access to the marketplace and improved patient outcomes.
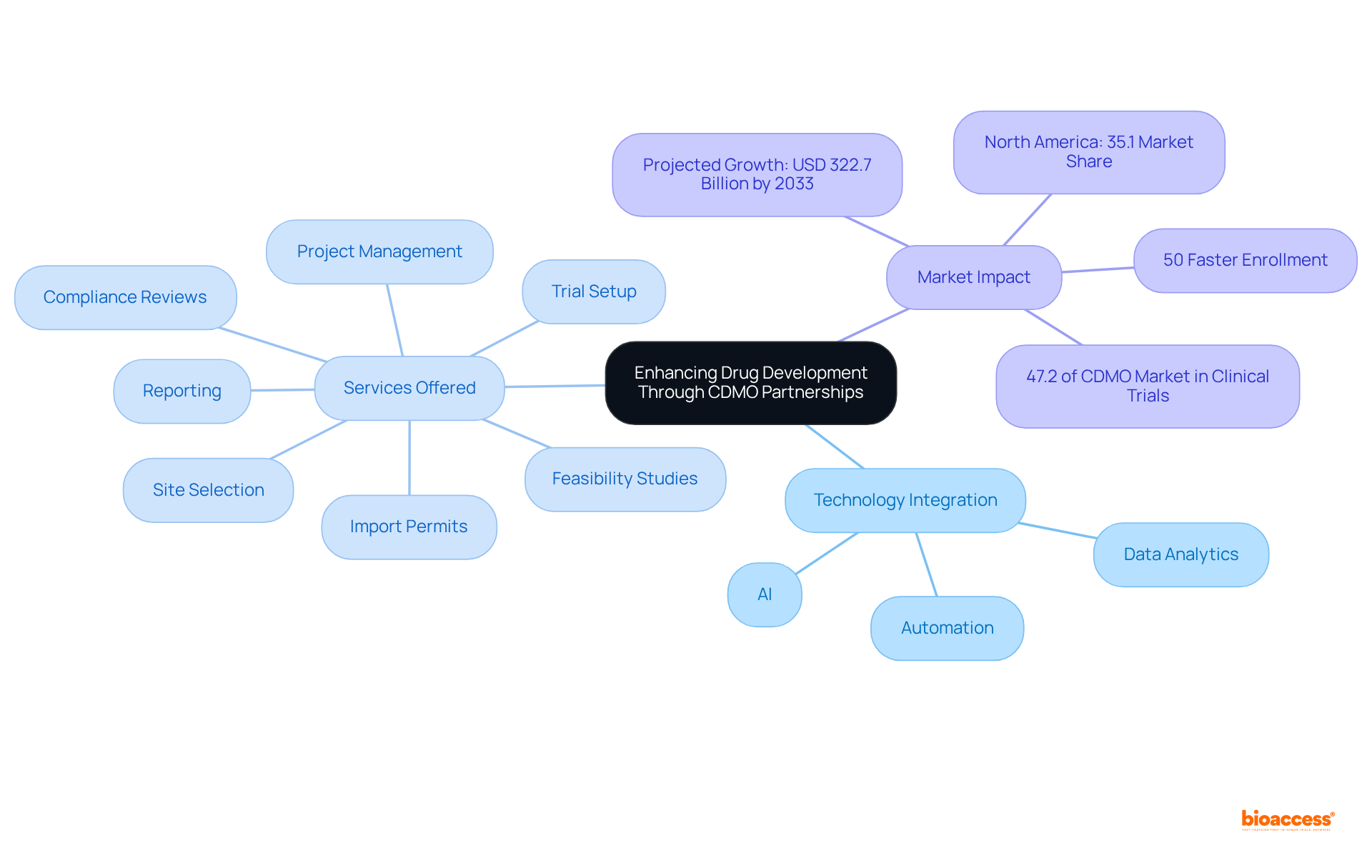
Collaborating with CDM pharma provides a crucial competitive edge by significantly accelerating time to market. Their specialized expertise in navigating complex regulatory pathways and optimizing production processes enables drug developers to launch products far more swiftly than traditional methods allow. This rapid pace is vital in the dynamic pharmaceutical landscape, where being the first to launch can yield substantial financial rewards. Indeed, companies that partner with CDM pharma frequently report improved financial outcomes, including enhanced EBITDA multiples and increased market share. As the industry continues to evolve, the ability to swiftly deliver innovative therapies to patients not only addresses urgent healthcare needs but also strategically positions companies within a competitive marketplace.
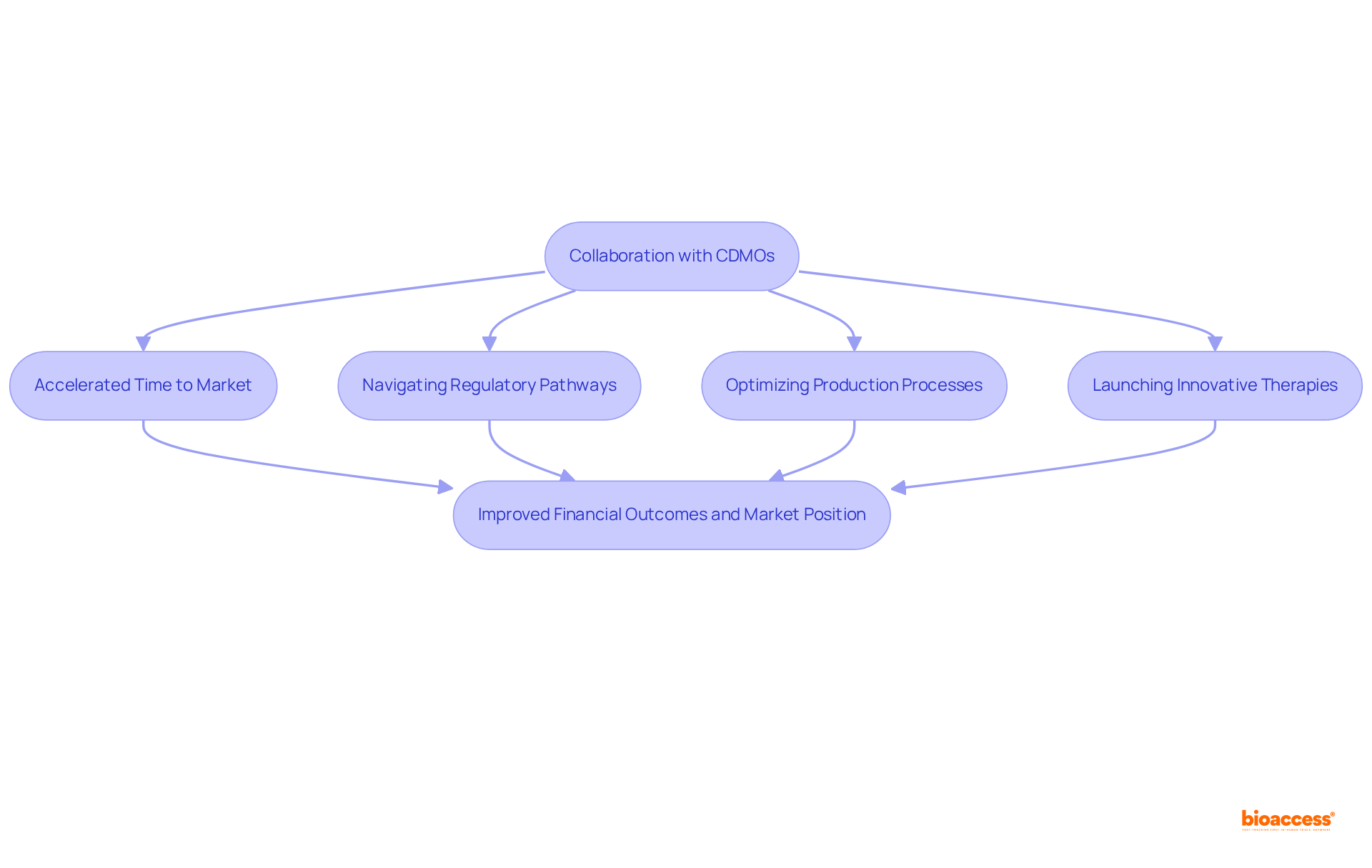
CDM Pharma provides a comprehensive range of services that span the entire product creation lifecycle, from preclinical research to commercialization. These services offered by CDM Pharma include:
This provides a streamlined solution for pharmaceutical companies. This integrated approach enhances the medicine creation process, allowing innovators to focus on advancing medical breakthroughs.
The Pharmaceutical CDMO 2.0 sector is projected to grow at a CAGR of 8.4% from 2025 to 2034, driven by the demand for integrated, technology-driven capabilities. By leveraging these services, CDMOs significantly improve pharmaceutical creation efficiency, ensuring seamless project advancement at every stage in CDM Pharma.
Notably, bioaccess® stands out by securing ethical approvals in just 4-6 weeks, activating over 50 pre-qualified sites in under 8 weeks, and achieving enrollment rates that are 50% faster than traditional markets, all while maintaining compliance with FDA, EMA, and MDR standards. This underscores bioaccess®'s distinct value in accelerating medical innovations and supporting medtech and biopharma startups across Latin America, Eastern Europe, and Australia.
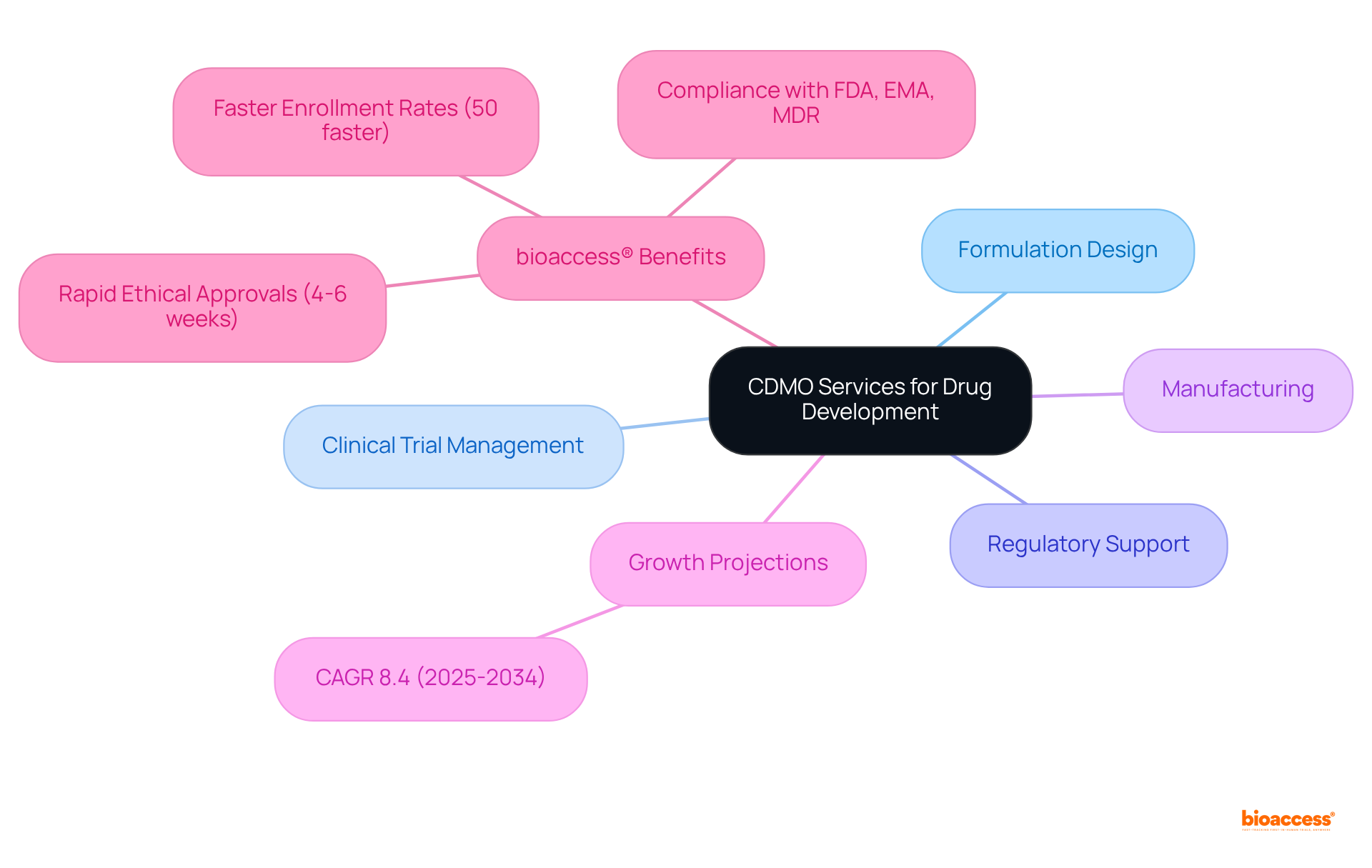
Startups and small biotech firms frequently encounter substantial hurdles in pharmaceutical innovation, primarily due to limited resources and expertise. By collaborating with CDM pharma, which includes Contract Development and Manufacturing Organizations (CDMOs), these companies gain access to critical infrastructure, specialized knowledge, and extensive support, significantly accelerating their growth trajectory.
CDMOs offer customized solutions that empower startups to navigate the intricate landscape of pharmaceutical development effectively. This partnership allows them to focus on their innovative ideas while ensuring that their projects are executed with precision and efficiency.
For instance, the oncology sector, which is estimated to account for approximately 35% of CDMO activity, illustrates how small biotech companies can leverage CDMO expertise to expedite the introduction of targeted therapies.
Furthermore, the U.S. pharmaceutical CDMO market, driven by cdm pharma, is projected to expand from USD 36.77 billion in 2024 to USD 68.57 billion by 2034, underscoring the increasing dependence of small biotech firms on CDMO partnerships to enhance their operational capabilities and streamline drug development processes.
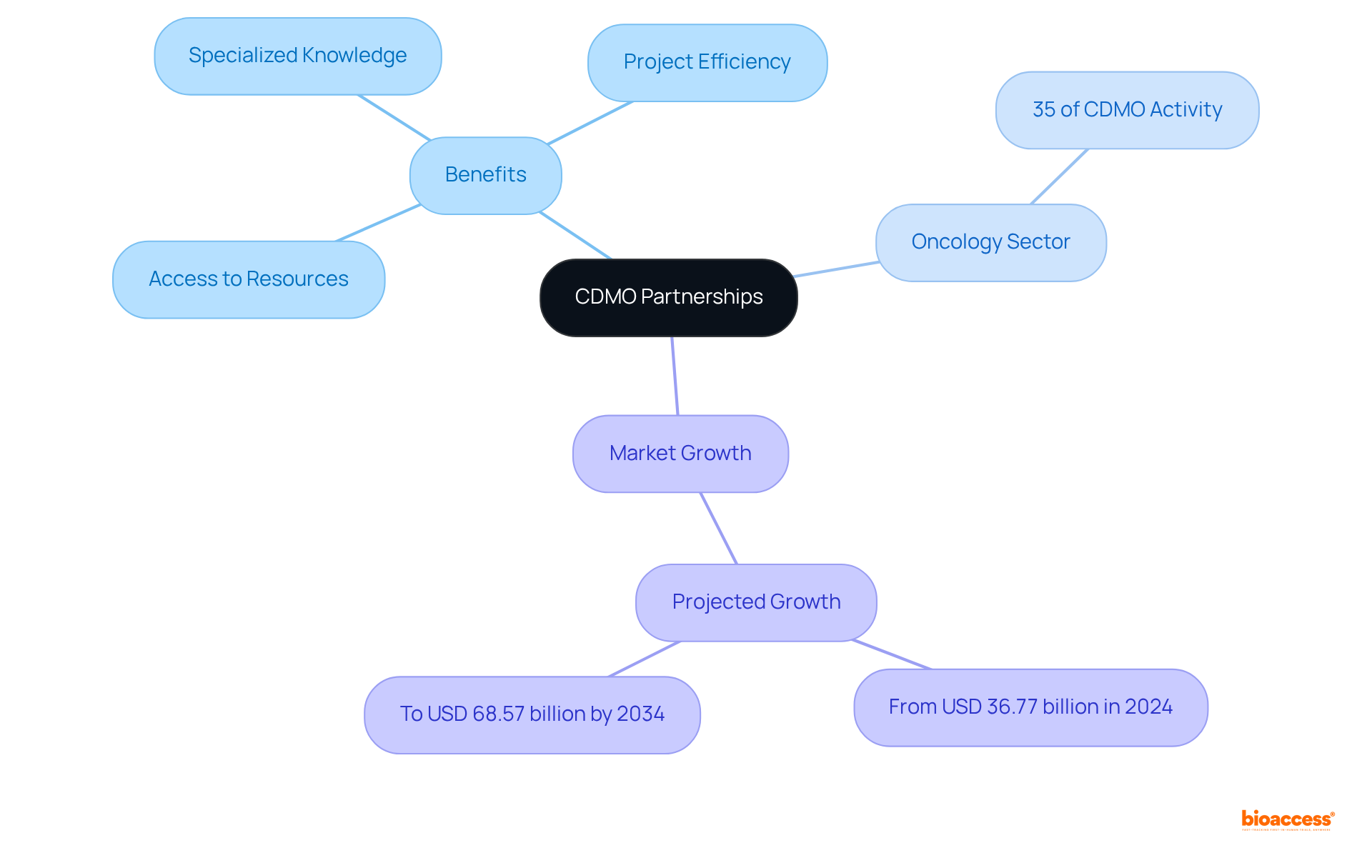
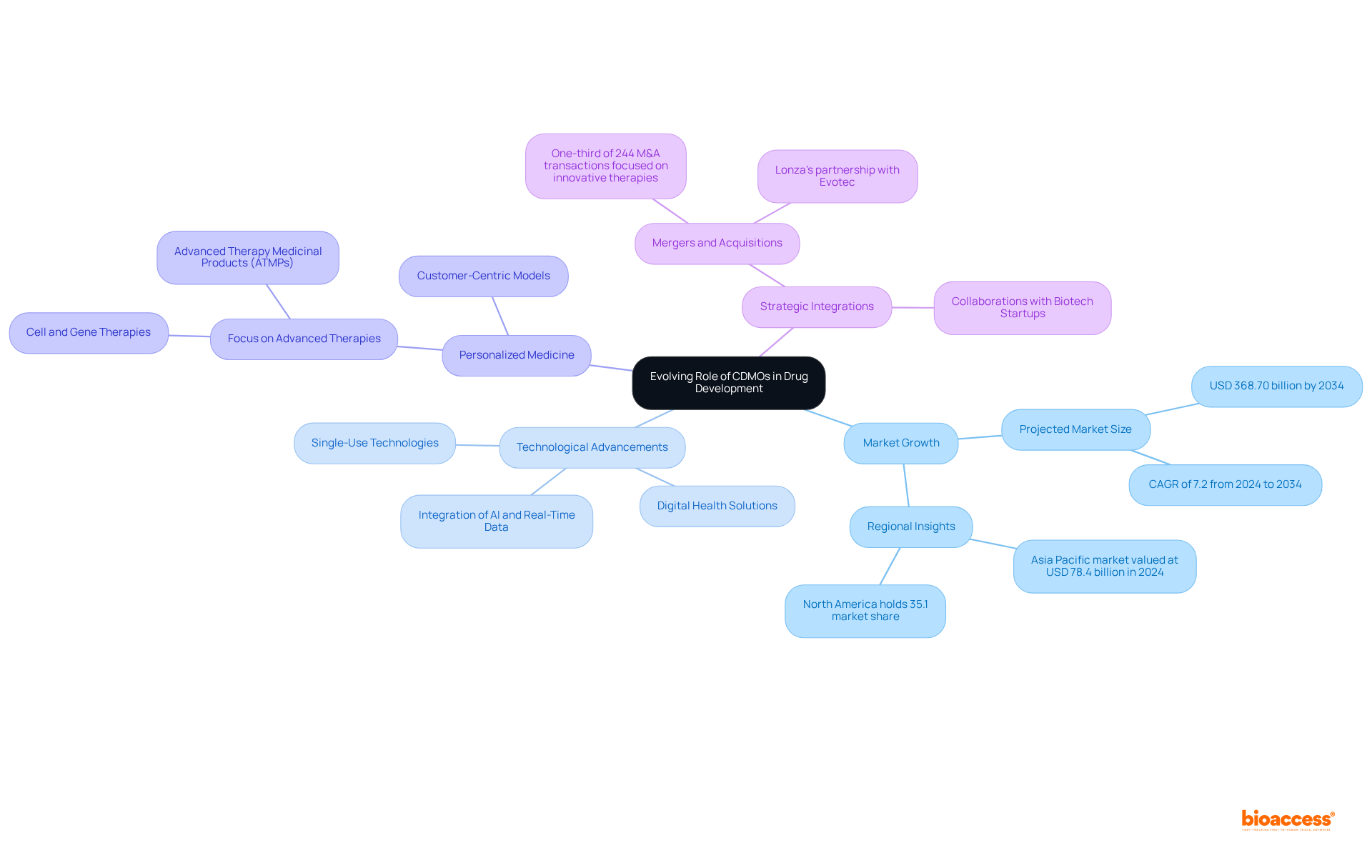
The pharmaceutical sector is undergoing significant transformation, which is concurrently reshaping the role of CDM pharma organizations. With a growing emphasis on personalized medicine and advanced therapies, CDM Pharma companies are increasingly tasked with enhancing their capabilities and expanding their service offerings. This evolution is essential for cdm pharma to maintain their position as indispensable partners in drug development within a complex and swiftly changing environment.
Statistics reveal that the global pharmaceutical CDMO market, which includes cdm pharma, is anticipated to grow at a compound annual growth rate (CAGR) of 7.2% from 2024 to 2034, potentially reaching around USD 368.70 billion by 2034. This expansion is propelled by the escalating demand for advanced therapies, such as cell and gene therapies, which necessitate specialized manufacturing competencies in cdm pharma.
In response to these trends, CDM pharma companies are integrating digital health solutions and personalized medicine into their service models. For example, organizations like Fujifilm Diosynth Biotechnologies and Lonza are making substantial investments in biomanufacturing to satisfy the rising demand for biologics and advanced therapy medicinal products (ATMPs). Furthermore, the emergence of novel modalities has resulted in a marked uptick in mergers and acquisitions within the sector, with one-third of the 244 M&A transactions from 2017 to 2021 concentrating on innovative therapies.
Moreover, cdm pharma companies are harnessing advanced technologies such as AI and real-time data exchange to optimize operations and improve efficiency. This digital transformation is vital for navigating the complexities of modern pharmaceutical development, allowing contract manufacturing organizations to provide comprehensive support throughout the entire production process, from initial formulation to market launch.
As the landscape of pharmaceutical development continues to evolve, cdm pharma organizations are positioned to play a pivotal role in facilitating the successful introduction of new therapies, ensuring they remain at the forefront of innovation in the industry.
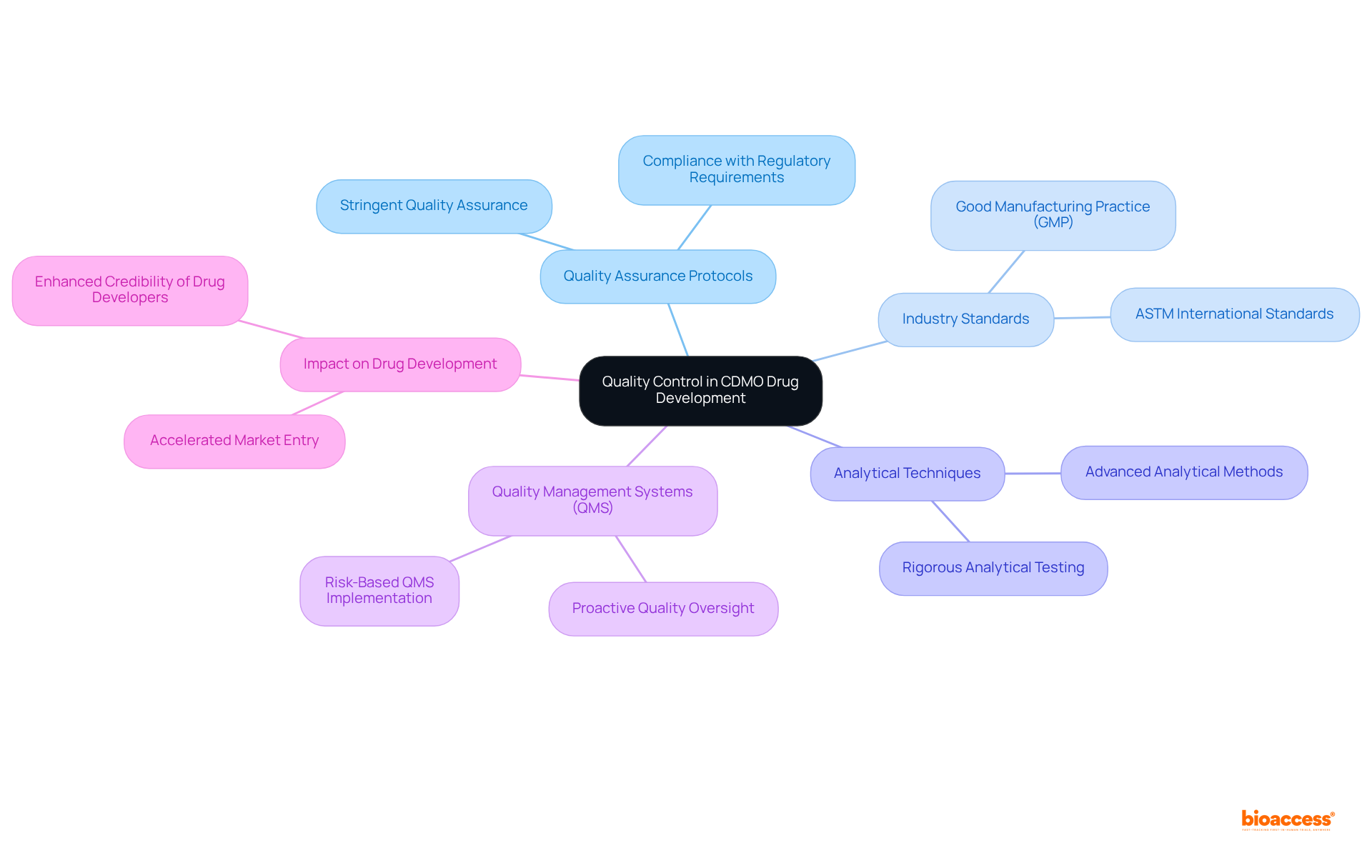
Quality control is paramount in pharmaceutical development, with CDM Pharma playing a vital role in ensuring excellence throughout this process. By implementing stringent quality assurance protocols and adhering to established industry standards, CDMOs guarantee that all products consistently meet the highest quality benchmarks. This unwavering commitment to quality not only bolsters the credibility of drug developers but also safeguards patient safety and ensures compliance with regulatory requirements.
For instance, CDMOs employ advanced analytical techniques and uphold Good Manufacturing Practice (GMP) standards, which are essential for producing reliable and effective pharmaceutical products. Furthermore, the incorporation of robust Quality Management Systems (QMS) enables CDMOs to proactively oversee quality at each phase of development, transforming compliance into a competitive advantage.
As the pharmaceutical landscape continues to evolve, the role of CDM Pharma in enhancing drug quality assurance becomes increasingly critical, ultimately accelerating the journey from development to market.
The role of CDM Pharma in enhancing drug development efficiency is increasingly critical in today's fast-paced pharmaceutical landscape. By leveraging strategic partnerships with Contract Development and Manufacturing Organizations (CDMOs), companies can expedite their processes, optimize costs, and navigate complex regulatory environments more effectively. This collaboration not only accelerates time to market but also ensures that innovative therapies reach patients who need them most.
Key insights throughout the article highlight how CDMOs enhance various aspects of drug development:
These advantages of working with CDMOs are clear. Furthermore, the importance of these organizations in maintaining high standards while driving innovation is underscored.
As the pharmaceutical industry continues to evolve, embracing the capabilities offered by CDMOs is essential for companies looking to thrive. By fostering these partnerships, drug developers can not only improve their operational efficiency but also contribute to a more agile and responsive healthcare system. The future of drug development lies in collaboration, and seizing the opportunities presented by CDMOs can pave the way for groundbreaking advancements in patient care.
What is bioaccess and how does it benefit drug development?
bioaccess® accelerates drug development by leveraging the regulatory speed of Latin America, diverse patient populations in the Balkans, and efficient ethical approval pathways in Australia, enabling approvals in 4-6 weeks and facilitating faster market access.
How much faster are enrollment rates with bioaccess compared to traditional markets?
Enrollment rates with bioaccess® are 50% faster than those in traditional markets, allowing companies to deliver their innovative products to patients sooner.
What cost savings can companies achieve by conducting trials in Colombia?
Companies can achieve cost savings exceeding 30% by conducting trials in Colombia compared to North America, with comprehensive reviews completed in 90-120 days.
How do CDMOs optimize pharmaceutical budgets?
CDMOs optimize pharmaceutical budgets by providing cost-efficient solutions tailored to drug developers' needs, leveraging economies of scale and specialized expertise to reduce operational expenses.
Why are CDMOs particularly beneficial for startups and small biotech firms?
CDMOs are beneficial for startups and small biotech firms as they help these companies operate within constrained budgets by offering high-quality services at competitive rates.
What is the significance of the salary disparity for professionals in Taiwan's CDMO sector?
The salary disparity allows CDMOs in Taiwan to offer services at lower costs, as professionals often earn significantly less than their U.S. counterparts, which aids in budget efficiency for drug developers.
How do CDMOs address capacity constraints in pharmaceutical production?
CDMOs address capacity constraints by providing the necessary infrastructure and expertise for efficient production scaling, enabling drug developers to meet increasing demand without compromising quality.
What specific areas do CDMOs optimize in drug development?
CDMOs optimize processes for biologics and mRNA-based products, which require advanced manufacturing capabilities, ensuring that pharmaceutical companies can scale operations effectively.