


This article identifies eight essential regulatory software solutions that are pivotal for ensuring compliance in clinical research and various other industries. By spotlighting tools like bioaccess, OneTrust, and Fenergo, it illustrates how these solutions streamline regulatory processes, enhance operational efficiency, and ensure adherence to complex regulations. Ultimately, they empower organizations to maintain compliance while accelerating their operations.
In the ever-evolving Medtech landscape, the role of bioaccess is particularly significant. It addresses key challenges faced by organizations, providing insights that are crucial for navigating regulatory complexities. As compliance becomes increasingly critical, understanding how these software solutions can support your organization is vital.
Collaboration is essential in this context. By leveraging these regulatory software solutions, organizations can not only meet compliance requirements but also foster a culture of efficiency and innovation. The next steps involve evaluating these tools and considering how they can be integrated into your operational framework to enhance compliance and streamline processes.
The clinical research landscape is evolving rapidly, driven by the increasing need for efficiency and compliance in an ever-changing regulatory environment. Organizations are striving to expedite their clinical trials while adhering to stringent regulations, making the role of regulatory software solutions paramount.
This article explores eight essential regulatory software tools that not only streamline compliance processes but also enhance the overall success of clinical research initiatives.
How can these innovative solutions effectively address the challenges faced by researchers and organizations as they navigate complex regulatory frameworks?
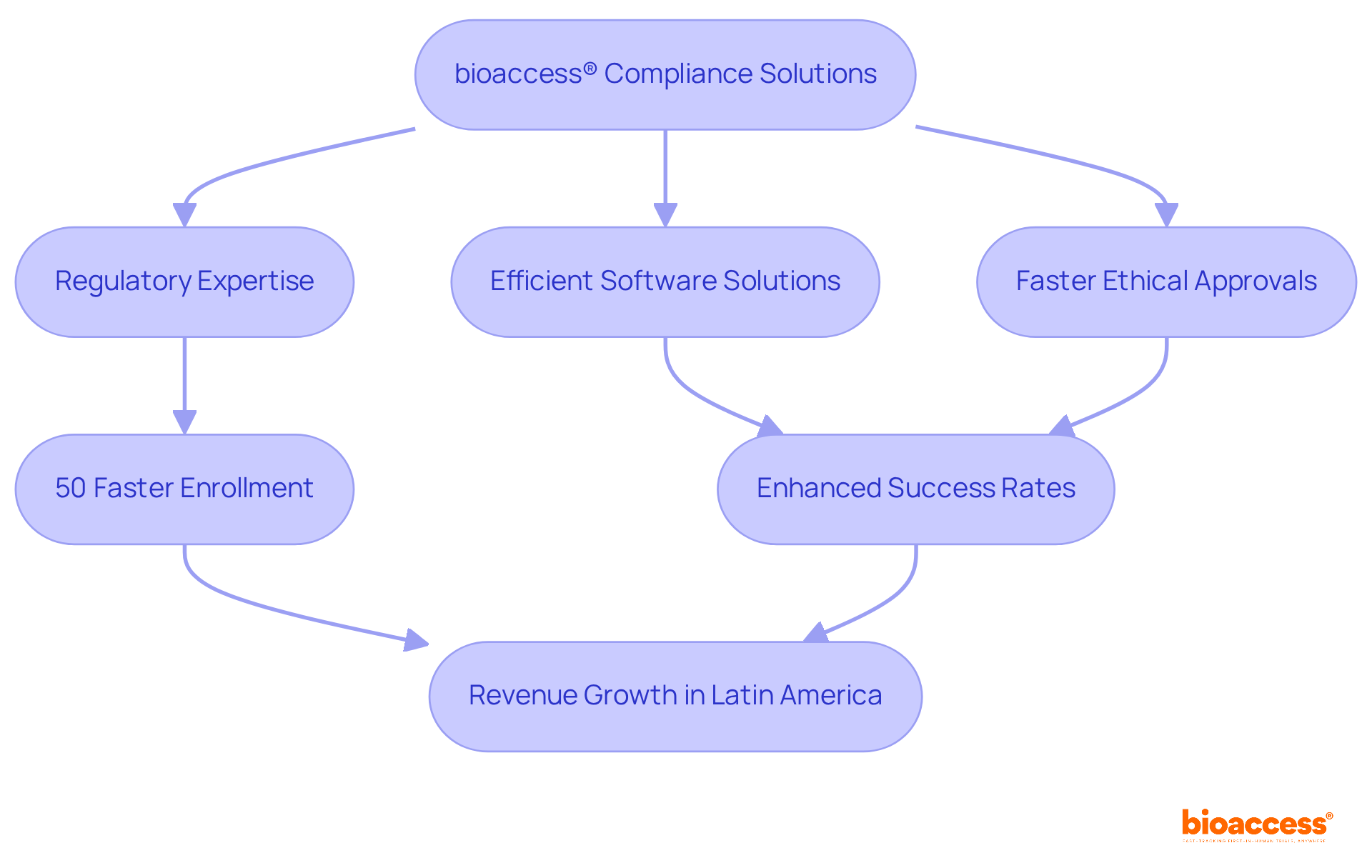
bioaccess® provides a robust framework designed to expedite clinical research through its efficient regulatory software solutions. With deep expertise in the regulatory landscapes of Latin America, the Balkans, and Australia, bioaccess® guarantees that clinical trials are conducted effectively and ethically. The organization has an impressive track record, delivering ethical approvals in just 4-6 weeks and achieving enrollment rates that are 50% faster than traditional markets. This remarkable efficiency not only accelerates the research process but also significantly enhances the overall success of clinical trials.
In 2024, the clinical trials market in Latin America generated a revenue of USD 1,432.1 million, with a projected compound annual growth rate (CAGR) of 7.9% from 2025 to 2033. This underscores the region's growing significance in global research. Successful clinical trials conducted in Latin America and Australia further illustrate bioaccess®'s capability to navigate complex regulatory landscapes with the aid of regulatory software while maintaining high standards of adherence. As the demand for innovative medical solutions continues to rise, bioaccess® emerges as an invaluable partner for Medtech, Biopharma, and Radiopharma innovators eager to accelerate their clinical research endeavors.
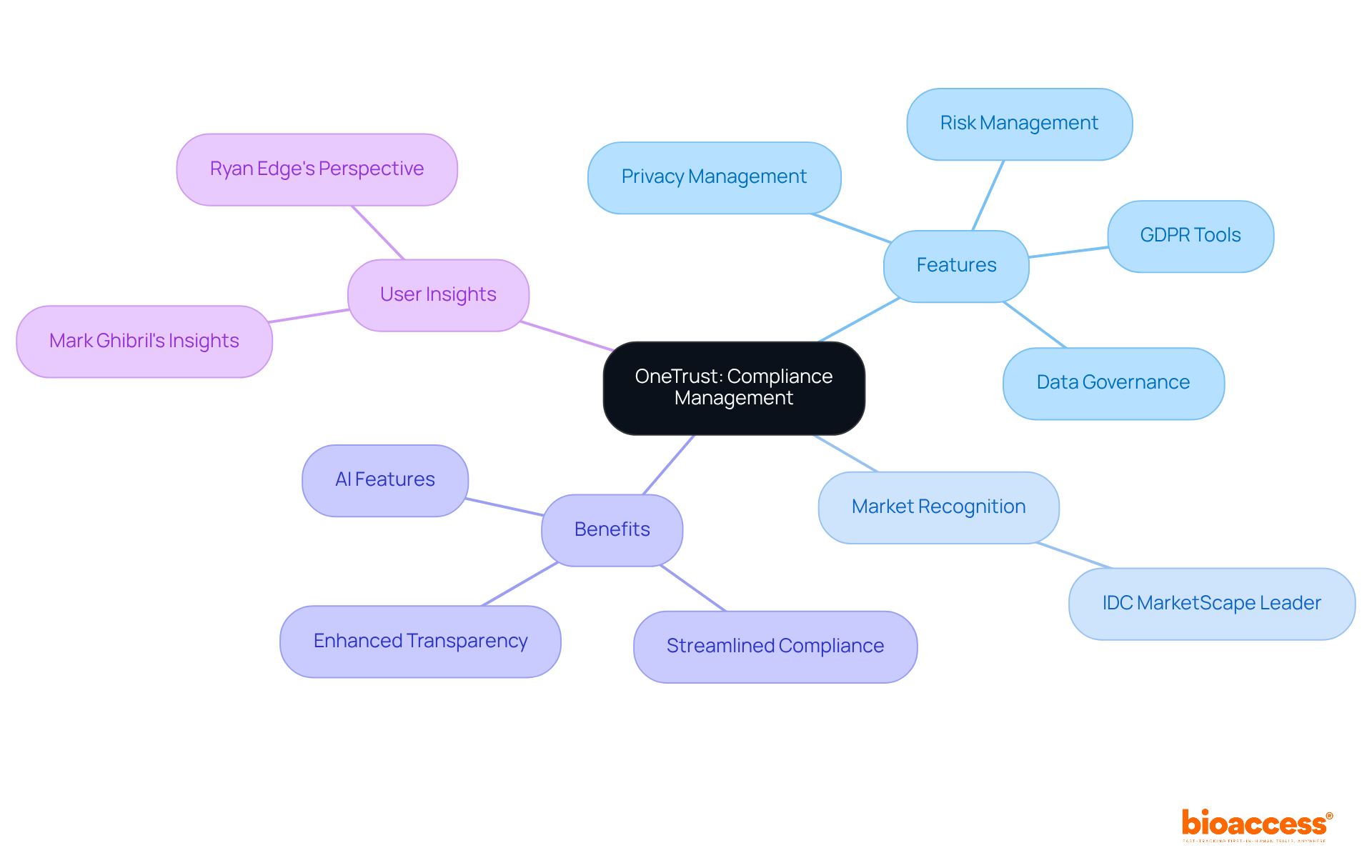
OneTrust stands out as a premier oversight platform, expertly guiding organizations through the intricate landscape of regulatory compliance with its regulatory software. With its powerful tools for privacy management, data governance, and risk management, it empowers companies to meet the demands of GDPR, CCPA, and other vital regulations. The regulatory software features a user-friendly interface that simplifies the regulatory process, while comprehensive reporting enhances transparency and accountability.
Recent upgrades to its GDPR compliance tools further bolster its effectiveness, making OneTrust an indispensable resource for clinical research organizations striving to streamline their regulatory efforts. By leveraging OneTrust's regulatory software, these entities can not only meet their regulatory requirements but also foster a culture of compliance that aligns with their operational goals.
Moreover, OneTrust has earned recognition as a Leader in the IDC MarketScape Worldwide Governance, Risk, and Compliance (GRC) Software 2025 Vendor Assessment, serving over 14,000 customers worldwide. This accolade highlights its reliability and strong market presence. Additionally, OneTrust's AI and automation features significantly enhance the productivity and effectiveness of GRC teams, amplifying its value proposition for organizations.
As Ryan Edge, Director of Privacy Automation, notes, "The idea of how to manage change related to regulations in the future is certainly one that businesses are contemplating now more than ever." This insight underscores the pressing need for robust compliance solutions, particularly regulatory software, in today's evolving regulatory environment.
Fenergo stands at the forefront of Know Your Customer (KYC) and Anti-Money Laundering (AML) regulation solutions, tailored specifically for financial institutions. Their advanced platform streamlines the onboarding process through automation, enabling organizations to efficiently verify customer identities and meet regulatory requirements. By leveraging Fenergo's solutions, financial institutions can significantly mitigate risks associated with regulatory breaches while enhancing their overall compliance framework.
This integration not only expedites customer onboarding but also equips institutions to adeptly navigate the complexities of ever-evolving regulatory landscapes. Current trends indicate a growing reliance on automated regulatory software solutions, as financial institutions strive to boost efficiency and accuracy in their KYC and AML processes. The adoption of such regulatory software technologies is essential for maintaining a competitive edge in the financial sector, ensuring that organizations can meet stringent regulatory standards while fostering trust with their clients.

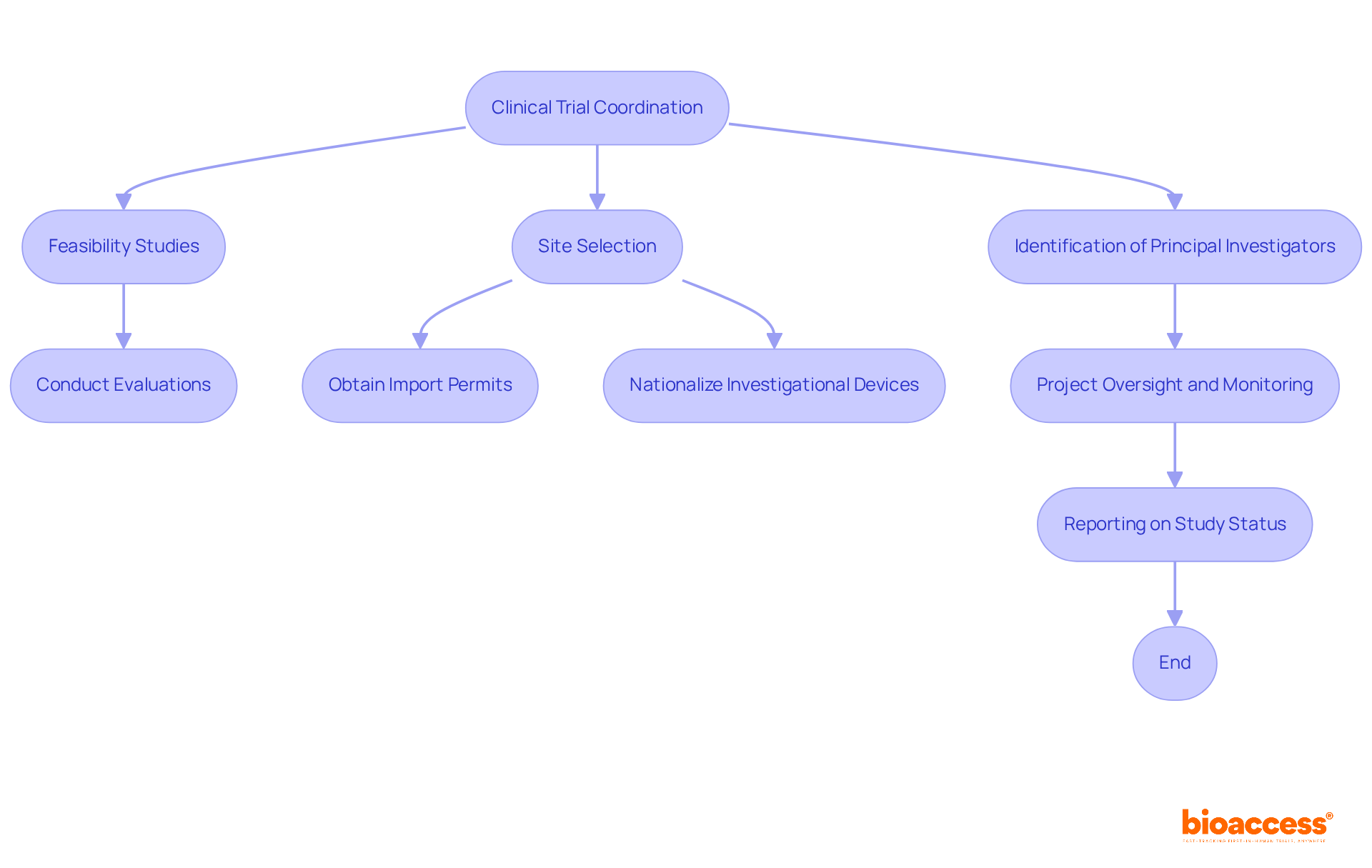
Bioaccess provides a comprehensive suite of clinical trial coordination services designed to streamline the research workflow while ensuring compliance with regulatory software standards. Our services encompass:
These services are all crucial for creating optimal conditions for clinical trials. We conduct thorough evaluations and offer insights on study documents to meet country-specific requirements, facilitating a smoother trial arrangement and approval process through ethics committees and health ministries.
Moreover, we assist in obtaining import permits and nationalizing investigational devices, ensuring that all legalities are meticulously addressed. Our dedicated project oversight and monitoring services keep studies on schedule, while our detailed reporting on study status, inventory, and adverse events guarantees transparency and accountability throughout the trial process.
The influence of Medtech clinical studies reaches far beyond immediate research outcomes; they play a significant role in boosting local economies by generating jobs, driving economic growth, and improving healthcare systems. By fostering international collaboration, Bioaccess is instrumental in cultivating a vibrant ecosystem that benefits both the research community and society as a whole.
VComply offers affordable governance, risk, and regulatory (GRC) solutions specifically designed for small and medium enterprises (SMEs). Their platform utilizes regulatory software to streamline regulatory management by providing essential tools for policy oversight, risk evaluation, and audit monitoring. This enables smaller organizations to effectively manage their regulatory responsibilities without the need for extensive resources. As regulatory demands are projected to rise in 2025, VComply emerges as an ideal choice for SMEs aiming to enhance their compliance efforts through regulatory software.
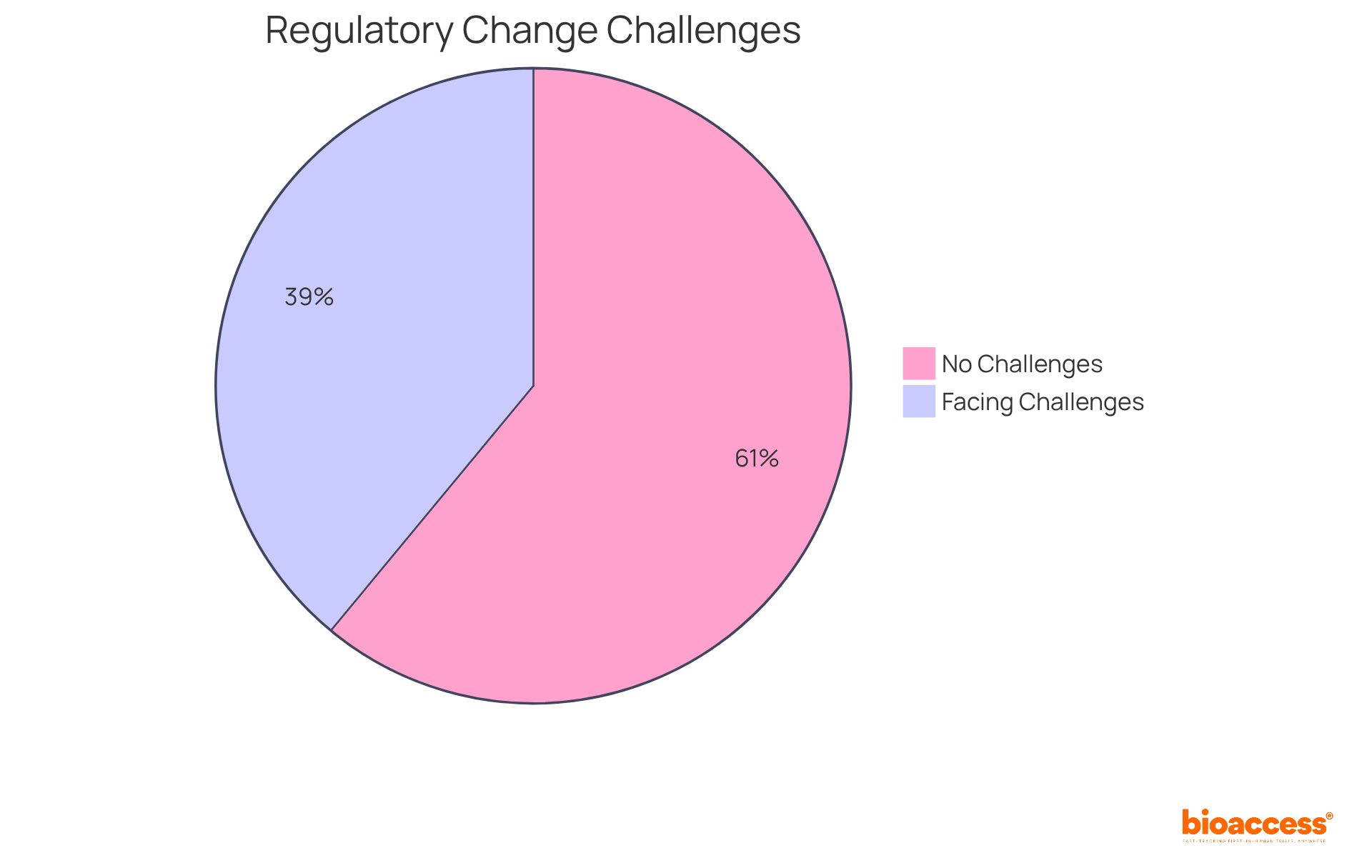
Notably, 39% of regulatory teams identify managing regulatory change as a significant challenge, underscoring the necessity for cost-effective regulatory software solutions like VComply to maintain adherence efficiency. Rick Stevenson, a former Manager of Compliance Advisory Services, noted, 'Our regulatory software automates your compliance procedures, ensuring you’re audit-ready regardless of which standard you need to follow.' Furthermore, organizations utilizing VComply, a type of regulatory software, have reported improved visibility into their compliance status, highlighting the platform's effectiveness in supporting regulatory oversight for small clinical research organizations.
To enhance your adherence oversight systems, consider exploring VComply's features today.
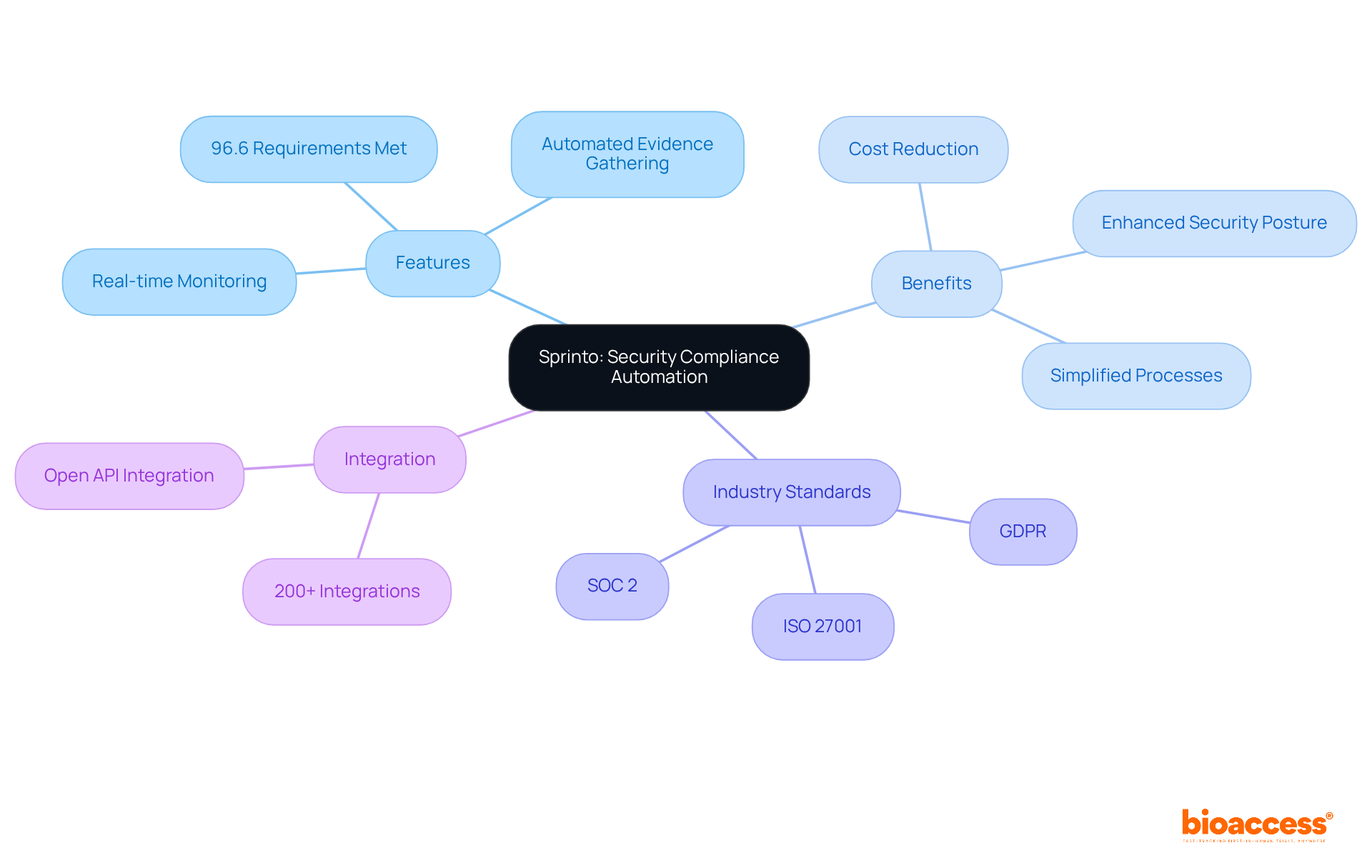
Sprinto stands out in the realm of security adherence automation, specifically designed for SaaS and IT firms, significantly streamlining the adherence process. Their platform simplifies regulatory oversight by using regulatory software to automate security evaluations and audits, enabling organizations to effectively meet industry standards such as ISO 27001 and SOC 2. Notably, leveraging automation tools like Sprinto can cut governance, risk, and regulatory (GRC) costs by 50%, presenting an economical solution for businesses. By adopting Sprinto, companies can bolster their security posture while reducing the time and effort required for managing regulatory software.
Moreover, Sprinto supports 96.6% of typical requirements right out of the box, showcasing its comprehensive capabilities. As organizations increasingly turn to automation for regulatory compliance—especially in the healthcare sector—regulatory software tools like Sprinto are becoming essential for maintaining a robust security stance and ensuring adherence to regulations. The platform's features, including real-time monitoring and automated evidence gathering, empower companies to proactively manage regulatory risks with regulatory software. As one expert noted, adherence is fundamentally about 'proving it, with time-stamped evidence that an auditor can trust.'
Additionally, Sprinto's integration capabilities ensure seamless compatibility with existing systems, enhancing its overall utility. For companies looking to embrace automation for regulatory compliance, evaluating regulatory software like Sprinto can significantly simplify processes and enhance adherence outcomes.
LogicGate provides no-code governance, risk, and regulatory software solutions specifically designed for large corporations. Their platform empowers organizations to customize regulatory workflows without the need for extensive programming skills, making it accessible for teams across various departments. By leveraging LogicGate, large entities can enhance their regulatory oversight and seamlessly adapt to changing legal requirements. This capability not only streamlines compliance processes but also positions organizations to respond proactively to evolving regulations.

Scrut Automation serves as a pivotal automation platform designed to optimize regulatory software processes for organizations. With robust features such as policy management, risk assessment, and audit tracking, it offers a comprehensive solution for navigating compliance across diverse regulations. By integrating Scrut Automation, organizations can significantly reduce manual efforts, enhance accuracy, and ensure adherence to industry standards.
In the ever-evolving landscape of regulatory compliance, the importance of regulatory software cannot be overstated. Organizations face mounting pressures to maintain compliance while managing complex regulatory requirements with the help of regulatory software. Scrut Automation not only streamlines these processes but also empowers teams to focus on strategic initiatives rather than getting bogged down in administrative tasks.
Ultimately, adopting regulatory software like Scrut Automation is not just a choice; it’s a strategic move towards operational excellence. Organizations that embrace this platform position themselves to thrive in a competitive environment, ensuring they remain compliant and ahead of the curve. Take the step today to transform your regulatory processes and experience the benefits firsthand.

The landscape of clinical research is evolving rapidly, and the integration of regulatory software solutions is proving to be a game-changer in enhancing efficiency and compliance. These tools streamline complex regulatory processes and empower organizations to navigate regulatory landscapes more effectively, ultimately leading to faster and more successful clinical trials. Companies like bioaccess, OneTrust, and Fenergo exemplify how innovative software can transform compliance management and accelerate research timelines.
Throughout this discussion, key insights into various regulatory software solutions have been provided, each tailored to meet the specific needs of different sectors. From bioaccess's robust clinical trial support to OneTrust's comprehensive compliance management and Fenergo's KYC and AML solutions, the importance of these tools cannot be overstated. Moreover, the rise of platforms like VComply and Sprinto demonstrates a growing trend toward affordable and automated compliance solutions, making regulatory adherence accessible to small and medium enterprises.
In conclusion, embracing regulatory software is not merely a tactical choice; it is essential for organizations striving to stay competitive and compliant in an increasingly complex regulatory environment. As the demand for innovative medical solutions and rigorous compliance continues to rise, investing in these technologies will be crucial for driving operational excellence and ensuring the success of clinical research initiatives. Organizations are encouraged to explore these solutions and adapt them to their specific needs, paving the way for a more efficient and compliant future in clinical research.
What is bioaccess® and what does it offer?
bioaccess® provides a framework designed to expedite clinical research through efficient regulatory software solutions, ensuring that clinical trials are conducted effectively and ethically.
How quickly can bioaccess® deliver ethical approvals for clinical trials?
bioaccess® can deliver ethical approvals in just 4-6 weeks.
What are the enrollment rates achieved by bioaccess® compared to traditional markets?
bioaccess® achieves enrollment rates that are 50% faster than those in traditional markets.
What is the significance of the clinical trials market in Latin America?
In 2024, the clinical trials market in Latin America generated a revenue of USD 1,432.1 million, with a projected compound annual growth rate (CAGR) of 7.9% from 2025 to 2033.
Who are the target clients for bioaccess®?
bioaccess® targets Medtech, Biopharma, and Radiopharma innovators looking to accelerate their clinical research endeavors.
What does OneTrust specialize in?
OneTrust specializes in comprehensive compliance management, guiding organizations through the regulatory compliance landscape with its regulatory software.
What features does OneTrust's regulatory software offer?
OneTrust's regulatory software includes tools for privacy management, data governance, risk management, and a user-friendly interface for simplifying the regulatory process.
How does OneTrust enhance transparency and accountability?
OneTrust enhances transparency and accountability through comprehensive reporting features within its regulatory software.
What recognition has OneTrust received in the compliance software market?
OneTrust has been recognized as a Leader in the IDC MarketScape Worldwide Governance, Risk, and Compliance (GRC) Software 2025 Vendor Assessment.
How many customers does OneTrust serve?
OneTrust serves over 14,000 customers worldwide.
What is the importance of AI and automation in OneTrust's offerings?
OneTrust's AI and automation features significantly enhance the productivity and effectiveness of Governance, Risk, and Compliance (GRC) teams, amplifying its value proposition for organizations.
Why is there a pressing need for robust compliance solutions?
There is a pressing need for robust compliance solutions due to the evolving regulatory environment, as businesses contemplate how to manage change related to regulations.