


The article titled "8 Key CSR Clinical Trials Insights for Research Directors" primarily aims to equip research directors with crucial insights into clinical study reports (CSRs) that can significantly enhance their trial processes.
It underscores the necessity of comprehending the various types of CSRs and advocates for the adoption of standardized templates.
Additionally, it highlights the advantages of leveraging specialized services to bolster compliance, improve patient recruitment, and streamline data management.
This strategic approach ultimately facilitates more efficient and successful clinical trials.
The landscape of clinical research is evolving rapidly, fueled by the increasing complexity of trials and the urgent need for efficiency. Research directors are confronted with the challenge of navigating this intricate environment while ensuring that their studies remain compliant and effective. This article explores eight key insights designed to empower these leaders to optimize their clinical trials, from leveraging innovative platforms like bioaccess® to implementing robust data management practices. How can research directors adapt to these changes and enhance their trial outcomes in a competitive market?
bioaccess® distinguishes itself in the clinical research landscape by leveraging the regulatory agility of Latin America, the diverse patient demographics in the Balkans, and the efficient pathways in Australia. This strategic combination facilitates ethical approvals in an impressive 4-6 weeks and accelerates enrollment by 50% compared to traditional markets.
As the clinical studies sector is projected to reach approximately USD 886.5 billion in 2025, study leaders can harness this flexibility by partnering with bioaccess® to optimize their trials. This collaboration ensures that innovative therapies reach the market more swiftly and effectively.
Given that the average enrollment pace in research is frequently hindered by delays, bioaccess®'s approach not only mitigates these challenges but also enhances the overall success rates of investigations, establishing it as an indispensable ally in the advancement of medical research.

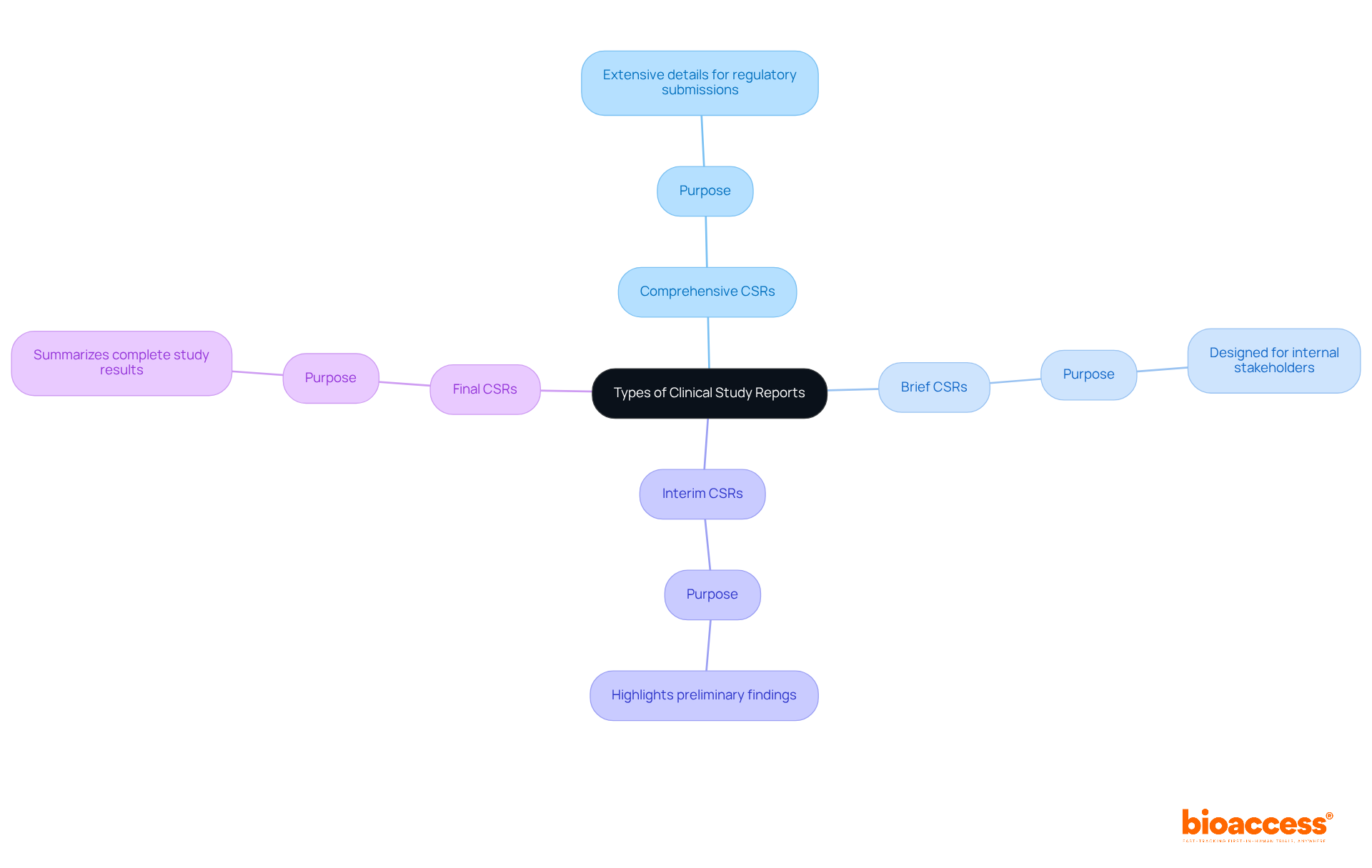
CSR clinical trials are essential documents that encapsulate the methodology and outcomes of clinical research reports. There are four primary types of CSRs:
Research indicates that a significant percentage of project directors prioritize detailed CSR clinical trials for their thoroughness and adherence to regulatory standards. Understanding these report categories empowers project leaders to customize their documentation, effectively addressing the needs of diverse stakeholders and ultimately enhancing the clarity and efficacy of their medical study communications.
To further augment the effectiveness of CSR clinical trials, research directors should consider implementing a standardized template that aligns with regulatory expectations and facilitates easier updates throughout the study process. Additionally, leveraging comprehensive research study management services, such as those offered by bioaccess, can significantly streamline the CSR process. These services encompass feasibility assessments, site selection, compliance evaluations, research preparations, import permits, project oversight, monitoring, and reporting, ensuring meticulous management and documentation of all facets of the research project. This holistic approach not only fosters the creation of high-quality CSRs but also aligns with the regulatory excellence mandated by authorities like INVIMA, Colombia's National Food and Drug Surveillance Institute.
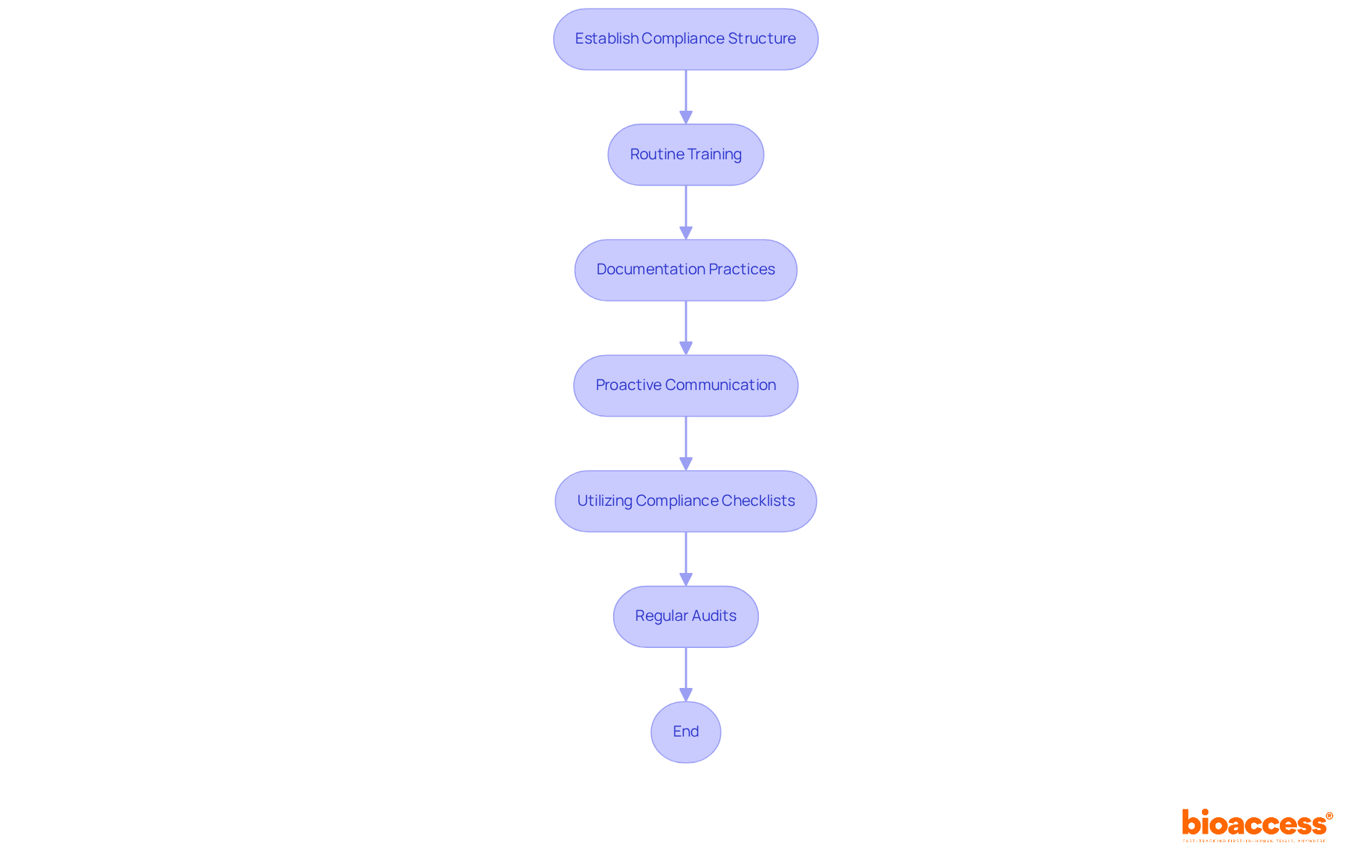
To ensure regulatory adherence, directors must establish a comprehensive compliance structure that incorporates routine training for study personnel on the latest regulations. Studies reveal that continuous education significantly improves compliance effectiveness, thereby reducing the risk of violations. Essential documentation practices provide a clear audit trail and facilitate accountability.
Furthermore, proactive communication with regulatory bodies is crucial, as it fosters transparency and trust. Utilizing tools such as compliance checklists can streamline adherence to guidelines, while regular audits identify potential gaps in compliance. This proactive approach not only safeguards the integrity of the research but also accelerates the approval process, ultimately enhancing the overall success of medical studies.
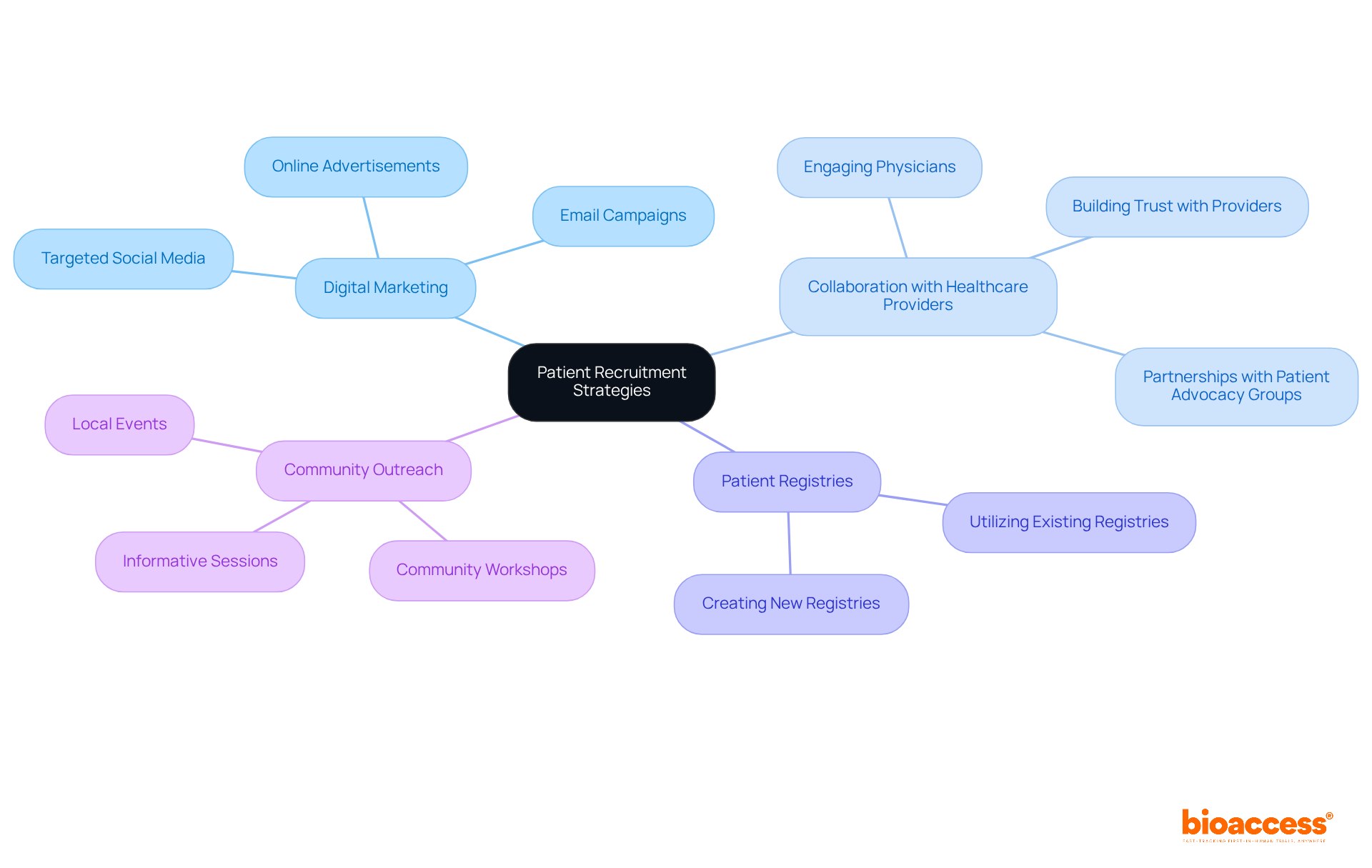
To enhance patient recruitment for research studies, research directors must adopt a comprehensive strategy that incorporates:
Digital marketing strategies, including targeted social media campaigns and online advertisements, can significantly broaden outreach, facilitating connections with potential participants actively seeking treatment options.
Engaging with nearby healthcare professionals is crucial, as many patients prefer obtaining information about research studies from their physicians. Establishing robust connections with these experts can enhance the credibility of studies and promote patient involvement. Moreover, collaborating with patient advocacy groups fosters trust and encourages participation, further amplifying recruitment efforts.
Community outreach initiatives also play a vital role in increasing awareness about clinical studies. By organizing community activities and informative workshops, project leaders can directly engage with prospective attendees, addressing their concerns and providing essential information about the studies. This holistic approach not only improves recruitment rates but also ensures that studies are representative of diverse populations, ultimately leading to more reliable and inclusive research outcomes.
Furthermore, initiatives like the partnership between bioaccess™ and Caribbean Health Group aim to position Barranquilla as a premier destination for medical studies in Latin America, with backing from Colombia's Minister of Health. Such collaborations can enhance the visibility and credibility of research studies in the region, further facilitating patient recruitment and involvement.
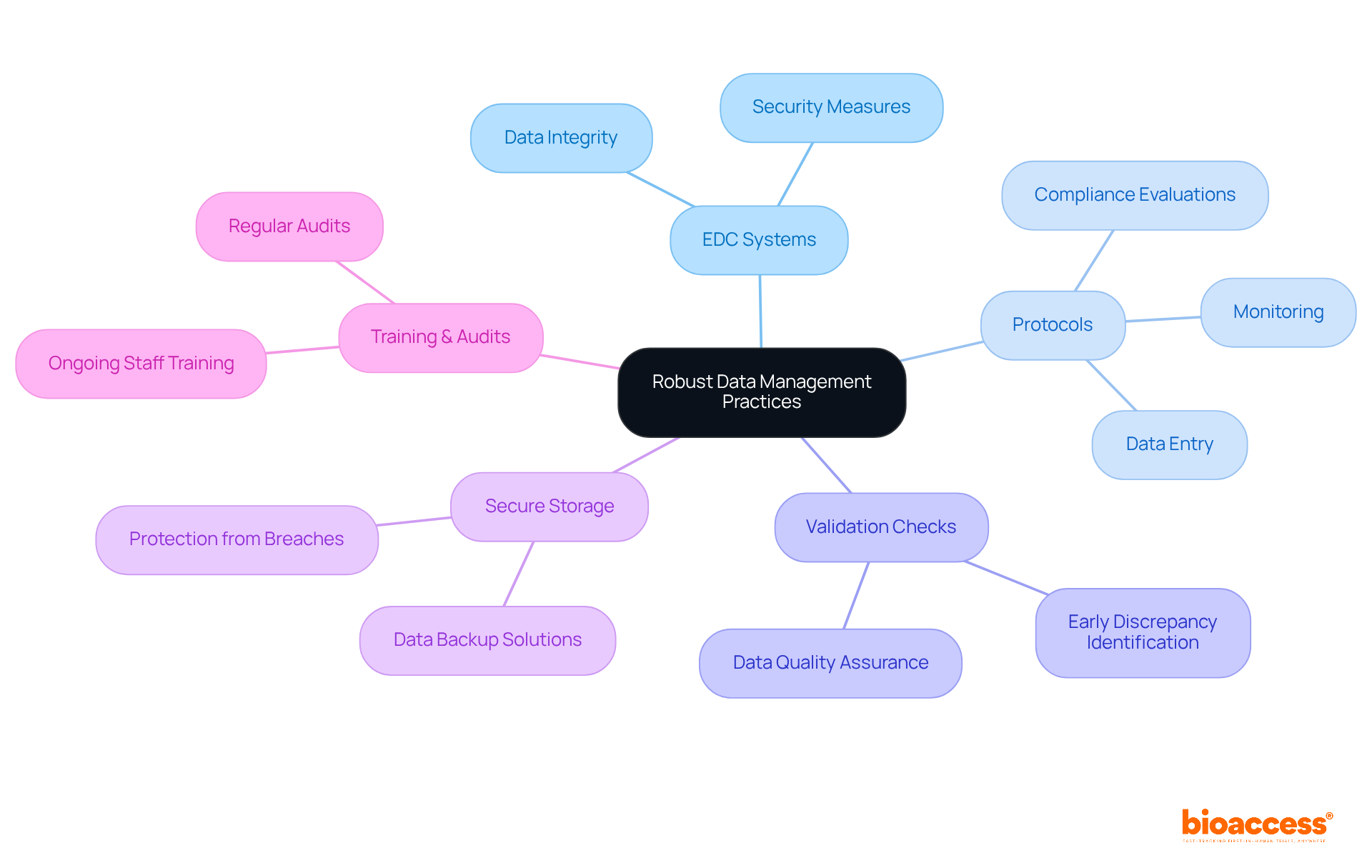
Efficient data management in medical studies is pivotal, hinging on the adoption of electronic data capture (EDC) systems that ensure data integrity and security. Research directors must prioritize the establishment of comprehensive protocols for data entry, monitoring, and analysis, which are essential to minimizing errors and enhancing data quality. Regular validation checks play a crucial role, facilitating the early identification of discrepancies and thereby safeguarding the integrity of the collected data. Additionally, maintaining secure data storage solutions is vital to protect sensitive information from breaches.
To bolster these practices, conducting regular audits and providing ongoing training sessions for staff can significantly improve data management outcomes. Utilizing extensive research management services provided by bioaccess—including:
can enhance the overall efficiency and effectiveness of research. Research indicates that organizations with robust data management frameworks experience fewer integrity issues, which can lead to more reliable results and faster regulatory approvals. By fostering a culture of continuous improvement and adherence to best practices, research directors can ensure that their clinical trials are not only compliant but also positioned for success in an increasingly data-driven landscape. This ultimately contributes to job creation, economic growth, healthcare improvement, and international collaboration.
Early-phase investigations, including first-in-human (FIH) and early-feasibility analyses (EFS), are crucial in the drug development process, providing essential preliminary data on safety and efficacy. In 2025, the significance of these investigations is underscored by their ability to identify potential issues early, thereby mitigating risks in subsequent development phases. Research directors are urged to prioritize FIH and EFS investigations, as they not only deepen the understanding of a product's performance but also enable informed decision-making.
Statistics reveal that early-phase research can profoundly affect the overall success of clinical trials. For instance, companies engaged in FIH research often experience a 50% faster enrollment rate compared to traditional methods, expediting their path to market. Collaborating with proficient contract research organizations (CROs) such as bioaccess, which provides essential services like regulatory dossier submission and principal investigator selection, can further enhance the design and execution of these initiatives, ensuring efficiency and effectiveness.
A prime example is Avantec Vascular's first-in-human clinical trial of an innovative vascular device in Latin America, supported by bioaccess. This partnership highlights the importance of leveraging expert services in managing regulatory requirements and site selection, both of which are critical for the success of early-phase research. Additionally, the VENOS-3 study, aimed at gathering evidence on the safety and efficacy of the Velocity system for creating arteriovenous fistulas for hemodialysis access, emphasizes the vital role of early-feasibility assessments in addressing significant unmet medical needs, particularly among high-risk patient populations. By harnessing insights from FIH and EFS investigations, project directors can significantly improve the likelihood of successful outcomes in their medical trials.
Collaborating with contract research organizations (CROs) like bioaccess offers significant advantages for research directors aiming to optimize clinical study processes. With over 20 years of experience in Medtech, bioaccess excels in managing diverse research types, including:
By leveraging their specialized expertise and resources, bioaccess enhances testing efficiency and improves overall outcomes. Research directors should prioritize CROs that have a proven track record in their specific therapeutic areas, ensuring alignment with study objectives through effective communication channels. This partnership not only leads to improved timelines but also results in substantial cost reductions—often averaging 20-30% compared to internal studies.
Dushyanth Surakanti, Founder and CEO of Sparta Biomedical, highlighted his experience with bioaccess during its initial human study in Colombia, stressing the critical role of their expertise in enhancing data quality and integrity, which ultimately fosters innovation and accelerates the path to market.
Successfully navigating market access requires a comprehensive understanding of the healthcare landscape, particularly payer requirements and reimbursement processes. Engaging health economists early in the clinical trial process is essential for crafting compelling value propositions that resonate with stakeholders.
Statistics reveal that early payer engagement can significantly facilitate market entry; studies indicate that proactive communication with payers results in a 30% increase in successful reimbursement outcomes. As the healthcare landscape continues to evolve, remaining informed about payer requirements becomes increasingly vital. Recent trends underscore a growing focus on value-based contracting and the incorporation of real-world evidence in decision-making.
Specific services provided by bioaccess, such as budget-impact models for SUS inclusion and patient access mapping, illustrate how leveraging such insights can bolster market access strategies. By prioritizing these approaches, research directors can enhance the accessibility of innovative therapies for patients while ensuring a smoother path to market. Understanding the challenges presented by economic uncertainty and policy changes is crucial for navigating the current market access environment.
The incorporation of technological innovations such as artificial intelligence (AI) and blockchain is set to revolutionize clinical studies. Research directors must actively explore how these technologies can enhance patient recruitment, streamline data management, and improve study monitoring. For instance, AI can analyze vast datasets to identify suitable candidates for studies, significantly accelerating recruitment processes.
With bioaccess®, treatment-naive cardiology or neurology cohorts can be enrolled 50% faster than traditional Western sites, resulting in savings of $25K per patient with FDA-ready data—no rework, no delays. As Wing Lon Ng observes, "AI can simulate various testing scenarios, recommend optimal strategies, and surface key insights from massive datasets," underscoring AI's potential to enhance design processes through data-driven decision-making.
Furthermore, blockchain technology bolsters data management by ensuring secure, transparent, and tamper-proof records, essential for maintaining the integrity of medical data. This synergy of technologies not only enhances efficiency and reduces costs but also fosters greater patient involvement throughout the process.
Additionally, comprehensive research study management services—including feasibility assessments, site selection, compliance evaluations, study setup, import permits, project oversight, and reporting—are vital for overcoming patient recruitment challenges. As the healthcare landscape evolves, embracing these advancements will be crucial for study leaders aiming to enhance the efficiency and reliability of medical evaluations.
Ongoing education for study groups is essential for enhancing the quality of medical experiments. Study leaders must prioritize:
to ensure their teams are well-versed in the latest advancements in medical investigation. Successful training programs, particularly those aimed at professional development for junior faculty, have shown significant impacts on job retention and overall team effectiveness.
Encouraging team members to participate in industry conferences not only fosters knowledge sharing but also enhances collaboration, ultimately leading to improved outcomes. By investing in the ongoing education of research staff, organizations can adeptly navigate the complexities of clinical trials and achieve superior results.
The insights shared in this article underscore the critical role of strategic approaches in enhancing the efficiency and effectiveness of clinical trials. By concentrating on key areas such as regulatory compliance, patient recruitment, data management, and technological advancements, research directors can significantly improve trial outcomes and expedite the journey for innovative therapies to reach the market.
Throughout the discussion, several essential strategies were outlined:
Additionally, leveraging early-phase studies and embracing technological innovations are pivotal in successfully navigating the complexities of clinical research. These insights highlight the necessity for continuous education and adaptation in an evolving healthcare landscape.
Ultimately, the call to action for research directors is clear: by prioritizing these strategies, they can enhance the quality and efficiency of clinical trials. Embracing a collaborative approach with platforms like bioaccess® and investing in ongoing team education will not only foster regulatory compliance but also ensure that trials are representative and inclusive. The future of clinical research hinges on these proactive measures to meet the challenges ahead and deliver impactful medical advancements.
What is bioaccess® and how does it enhance clinical trials?
bioaccess® is a clinical research organization that utilizes the regulatory agility of Latin America, diverse patient demographics in the Balkans, and efficient pathways in Australia to facilitate ethical approvals in 4-6 weeks and accelerate enrollment by 50% compared to traditional markets.
What is the projected growth of the clinical studies sector?
The clinical studies sector is projected to reach approximately USD 886.5 billion by 2025.
How does bioaccess® help in overcoming enrollment delays in clinical trials?
bioaccess® mitigates enrollment delays by providing a flexible and efficient approach, enhancing the overall success rates of clinical investigations.
What are the four primary types of Clinical Study Reports (CSRs)?
The four primary types of CSRs are: 1. Comprehensive CSRs - extensive details for regulatory submissions. 2. Brief CSRs - designed for internal stakeholders. 3. Interim CSRs - highlighting preliminary findings during the study. 4. Final CSRs - summarizing complete study results.
Why are detailed CSRs important for project directors?
Detailed CSRs are prioritized by project directors for their thoroughness and adherence to regulatory standards, which helps in effectively addressing the needs of diverse stakeholders.
What practices can enhance the effectiveness of CSR clinical trials?
Implementing standardized templates that align with regulatory expectations and utilizing comprehensive research study management services can enhance the effectiveness of CSR clinical trials.
How can directors ensure regulatory compliance in clinical research?
Directors can ensure regulatory compliance by establishing a comprehensive compliance structure, providing routine training for study personnel, maintaining essential documentation practices, and fostering proactive communication with regulatory bodies.
What tools can help streamline adherence to regulatory guidelines?
Compliance checklists and regular audits can help streamline adherence to regulatory guidelines and identify potential gaps in compliance.