


The article primarily addresses the critical changes to the FDA Quality System Regulation (QSR) that clinical research directors must grasp in anticipation of the transition to the Quality Management System Regulation (QMSR) in 2026. It emphasizes a shift towards a more integrated documentation approach, highlighting the increased focus on risk oversight throughout the product lifecycle. Additionally, it underscores the necessity for proactive compliance strategies, which are essential for manufacturers to uphold product safety and efficacy in a competitive regulatory landscape.
The impending changes to the FDA Quality System Regulation (QSR) herald a transformative shift for manufacturers, as the new Quality Management System Regulation (QMSR) is poised to take effect on February 2, 2026. This transition not only aligns U.S. regulations more closely with international standards, such as ISO 13485:2016, but also introduces critical compliance challenges that have the potential to reshape the landscape of clinical research. As manufacturers prepare for heightened scrutiny and a more integrated approach to quality management, a pressing question emerges: how can they effectively navigate these changes to ensure compliance while maintaining their competitive edge?
Bioaccess delivers tailored solutions that empower companies to effectively navigate the new FDA QSR requirements. With over 15 years of experience, we ensure prompt adherence, significantly minimizing delays in product development and market entry. Our strategic approach integrates local expertise with global standards, enabling swift adaptation to the dynamic regulatory environment.
As the FDA extends the compliance period from one year to two years, our clients benefit from a more manageable transition, allowing them to concentrate on innovation while we manage the intricacies of regulatory compliance. This proactive strategy not only accelerates timelines but also elevates the overall standard of clinical research, positioning our clients for success in a competitive marketplace.
The FDA's Quality Management System Regulation (QMSR) is set to replace the existing FDA QSR on February 2, 2026. This transition signifies a notable change in regulatory standards, aligning closely with ISO 13485:2016, which encourages a more integrated approach to quality oversight. The QMSR aims to streamline compliance processes, reduce redundancies, and ultimately enhance product safety and efficacy. As part of this transition, manufacturers will face increased scrutiny in critical areas such as risk oversight and documentation practices.
Manufacturers have a two-year transition period to comply with the QMSR, effective from February 2024. Recent statistics reveal that a significant portion of manufacturers are presently preparing for these changes, acknowledging the importance of adjusting their control systems to meet the new requirements. The QMSR introduces various significant distinctions from the FDA QSR, such as an increased focus on risk oversight throughout the product lifecycle and the integration of Design for Excellence principles, which aim to embed excellence into products from the design stage.
Examples of manufacturers successfully adapting to the FDA QSR changes include those who have engaged in proactive training and gap analysis to identify necessary adjustments. The FDA has highlighted that while ISO 13485:2016 certification is advantageous, it does not exempt manufacturers from FDA inspections, underscoring the necessity for thorough adherence to the QMSR.
FDA officials have noted that this incorporation of ISO standards into the QMSR is a pivotal move towards harmonizing U.S. regulations with international practices, ultimately facilitating smoother market access for medical devices. As the sector prepares for the QMSR, remaining knowledgeable and proactive will be crucial for ensuring compliance and upholding high standards of oversight.
The alignment of FDA QSR with ISO 13485:2016 marks a pivotal transition towards a unified management framework. For producers, this represents a commitment to a more structured approach to assurance, aligning with international best practices. Such alignment not only streamlines compliance but also bolsters the credibility of products in global markets.
It is imperative for companies to revise their assurance systems to reflect these changes, ensuring adherence to both FDA QSR and ISO standards.
Starting February 2, 2026, the FDA will implement new inspection procedures that emphasize a comprehensive assessment of control systems. This transition from the Quality System Inspection Technique (QSIT) to a more integrated approach will focus on evaluating the effectiveness of control measures and risk management practices as outlined by FDA QSR.
Manufacturers are required to comply with the existing QS regulation until this date, making it essential for them to prepare in advance. To ensure readiness for these inspections, manufacturers must:
It is important to note that while the FDA QSR is incorporating ISO 13485 within the QMSR, manufacturers are not mandated to obtain ISO 13485 certification to comply with the FDA QSR.
Furthermore, manufacturers should label documents as 'confidential' before providing them to the FDA during inspections. This proactive approach is crucial, as companies that fail to demonstrate compliance may encounter significant regulatory challenges.
Experts advise organizations to commence their preparations early, focusing on enhancing the standard and comprehensiveness of their documentation to withstand the increased scrutiny anticipated during FDA inspections.

Beginning in February 2026, the FDA will undertake comprehensive examinations of records associated with management systems to ensure compliance with FDA QSR, encompassing management review reports, internal audit findings, and supplier audit documentation. Manufacturers must ensure that these records are meticulously maintained and readily accessible for inspection as required by FDA QSR. This proactive strategy is designed to mitigate risks associated with non-compliance with the FDA QSR and to enhance overall assurance practices.
Notably, the average number of FDA warning letters surged from 17 in the early 2000s to 304 in 2020, illustrating the increasing scrutiny from the FDA regarding the FDA QSR. Frequent deficiencies identified during FDA QSR inspections often stem from inadequate documentation, with system issues accounting for 34% of all citations from 2014 to 2016.
As Paul Koziarz, a regulatory adherence specialist, emphasizes, 'You must assess adherence not as a cost, but as a money saver.' Companies that prioritize robust documentation practices are better positioned to endure scrutiny under FDA QSR and uphold their reputations. For instance, organizations that have effectively navigated past inspections frequently underscore their commitment to comprehensive quality oversight records, which serve as a testament to their operational integrity.
As the FDA QSR continues to elevate standards for adherence, the importance of maintaining high-quality record-keeping cannot be overstated.
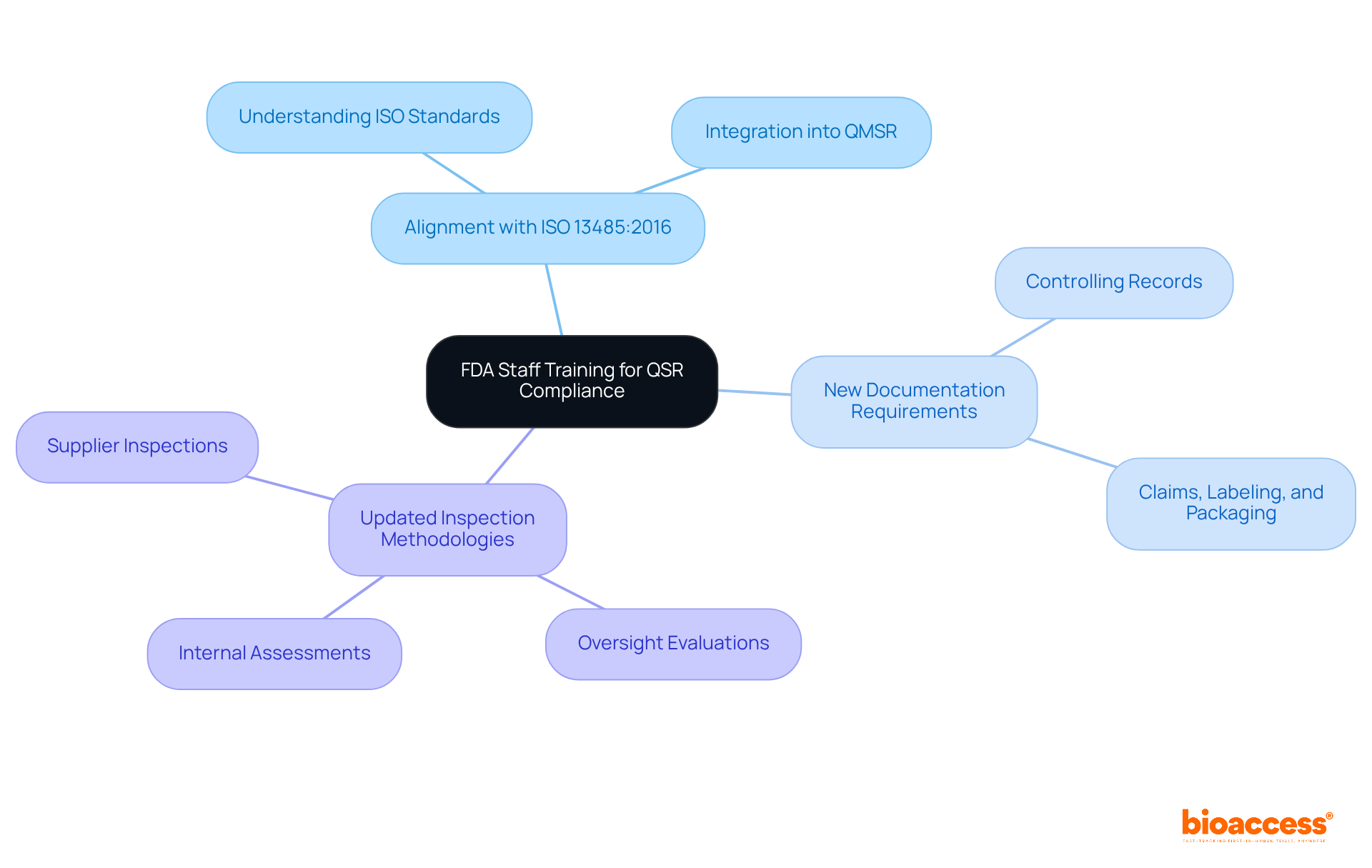
With the impending implementation of the Quality Management System Regulation (QMSR) on February 2, 2026, it is imperative that FDA personnel undergo comprehensive training to navigate the revised FDA QSR regulatory landscape and inspection protocols. This training will focus on several critical areas:
Notably, oversight evaluations, internal assessments, and supplier inspections will now fall under the scrutiny of the FDA QSR as mandated by the QMSR, making this training vital for equipping FDA personnel with the skills necessary to accurately assess compliance and assist manufacturers in adhering to the updated standards. As W. Edwards Deming aptly stated, "Excellence arises not from examination but from enhancement of the process," underscoring the importance of understanding these changes, which directly impact the efficacy of FDA compliance efforts and the overall assurance processes in the medical device industry.
The timeline for QMSR implementation encompasses several critical dates that manufacturers must prioritize:
As the enforcement date draws near, manufacturers are strongly urged to initiate preparations without delay. This necessitates revising performance control systems to align with the new requirements and ensuring that all personnel receive adequate training on these modifications. Recent statistics indicate that only 30% of manufacturers are currently on track to meet the QMSR adherence deadline, underscoring the imperative for proactive measures. Regulatory specialists emphasize that early planning is crucial; as one expert articulated, "By adopting the risk-based strategy and proactively aligning control systems with the new regulatory framework, manufacturers can position themselves for success in an increasingly complex and globalized market." Companies should prioritize conducting internal audits and gap analyses to evaluate their readiness for actual FDA QSR inspections, thereby ensuring they are well-prepared for the impending enforcement date. Furthermore, manufacturers should leverage the FDA's outreach and training initiatives during this transition period to enhance their understanding and implementation of the new requirements.

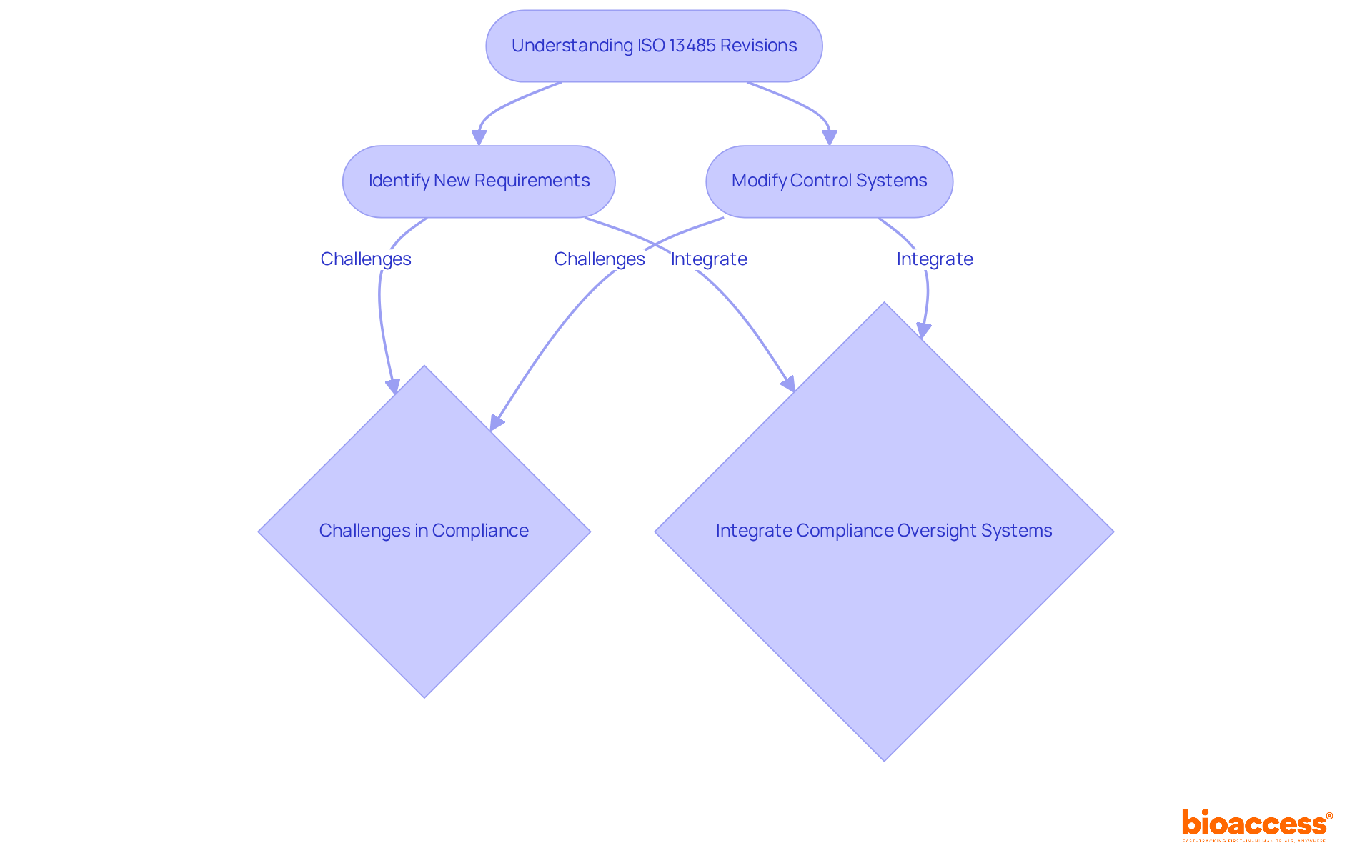
As ISO 13485 undergoes systematic reviews and potential revisions, manufacturers must remain proactive in understanding how these changes could influence their adherence to FDA QSR. Upcoming updates may introduce new requirements or modify existing ones, necessitating prompt adjustments to control systems. Approximately 90% of respondents in a recent survey expressed satisfaction with ISO 13485:2016, underscoring its effectiveness in aligning with regulatory frameworks.
However, many manufacturers encounter challenges in achieving full compliance due to misunderstandings of requirements and documentation difficulties. For instance, organizations that have successfully integrated automated compliance oversight systems have reported enhanced operational efficiency and reduced errors.
Staying informed about these changes is crucial for ensuring that products not only meet FDA QSR standards but also comply with international regulations, thereby facilitating easier market access and elevating product standards.
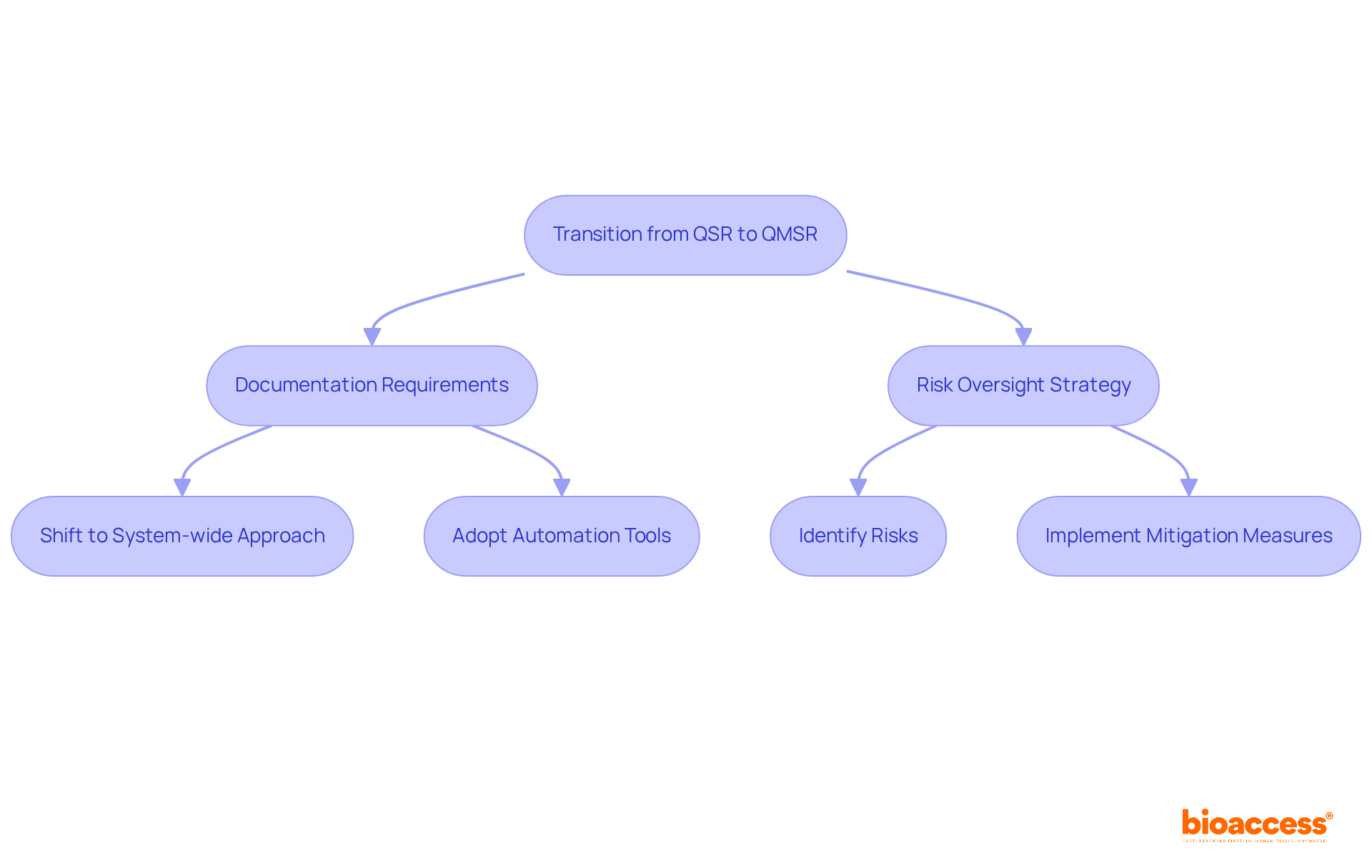
The transition from QSR to QMSR introduces several significant differences that clinical research directors must understand. Documentation requirements have shifted; whereas the FDA QSR emphasizes detailed FDA-specific records, QMSR focuses on a system-wide documentation approach. This change motivates producers to adopt a more comprehensive perspective of their assurance systems. Additionally, QMSR mandates a proactive risk oversight strategy throughout the product lifecycle. Manufacturers must not only identify risks but also implement effective measures to mitigate them.
To navigate these changes successfully, manufacturers should develop thorough adherence strategies that align their quality management systems with the new QMSR requirements. For instance, organizations can leverage automation tools to streamline documentation processes, ensuring that all necessary records are easily accessible. As highlighted by regulatory specialists, effective documentation strategies are essential for sustaining adherence rates and enabling smoother transitions to the new standards. By prioritizing these strategies, manufacturers can enhance their operational efficiency and compliance posture in the evolving regulatory landscape.
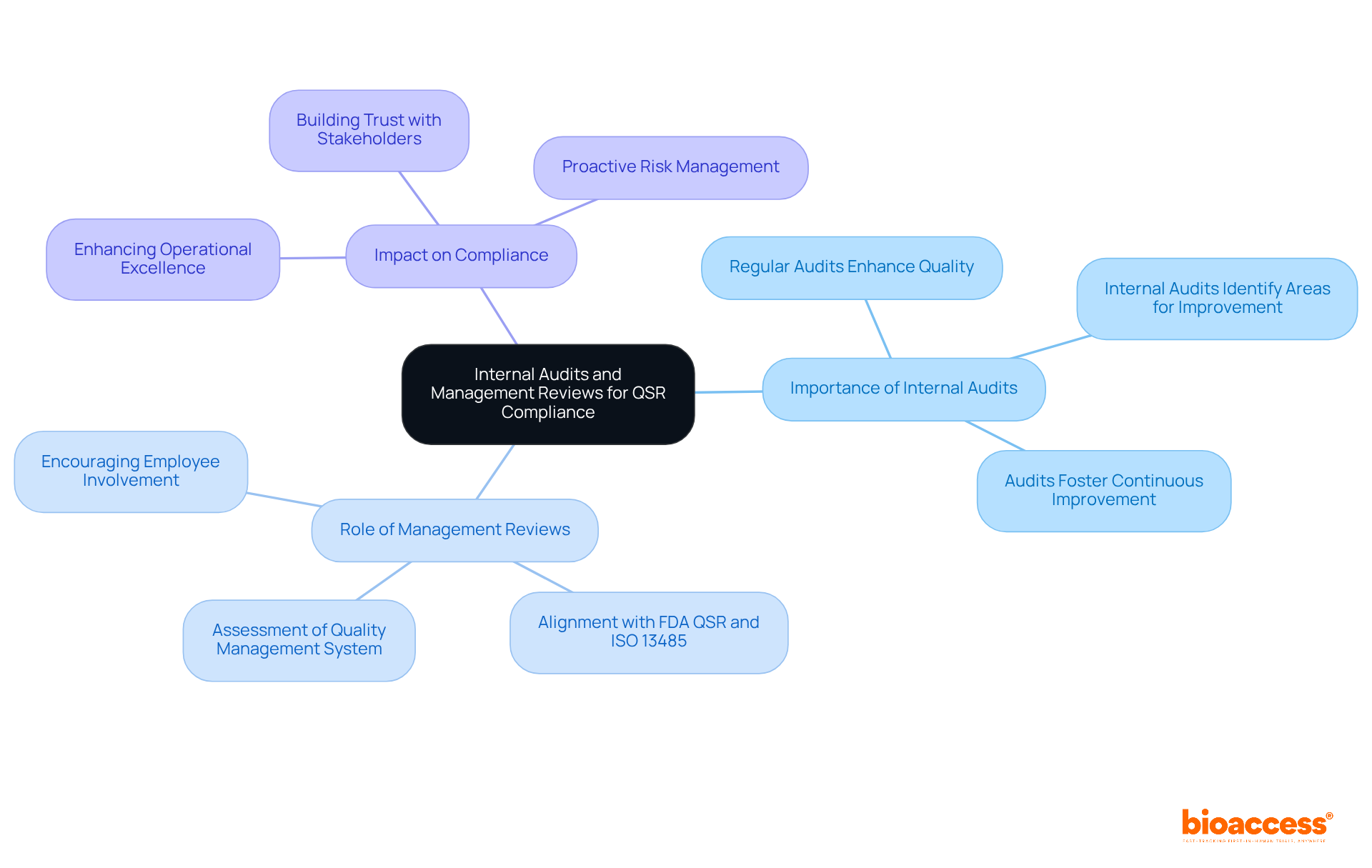
Internal audits and oversight evaluations are vital elements of an effective assurance system under the FDA QSR. Regular audits are crucial; studies reveal that only a small percentage of companies consistently perform these reviews, indicating a substantial opportunity for enhancement. Management reviews play a critical role in assessing the effectiveness of the quality management system, ensuring alignment with both FDA QSR and ISO 13485 standards. This proactive evaluation not only identifies areas for improvement but also fosters a culture of continuous enhancement within the organization.
As W. Edwards Deming stated, 'Quality comes not from inspection, but from improvement of the production process.' By adopting this philosophy, organizations can bolster compliance and propel operational excellence.
The impending changes to the FDA Quality System Regulation (QSR) signify a pivotal evolution in the regulatory landscape for clinical research directors and manufacturers alike. As the FDA transitions to the Quality Management System Regulation (QMSR), grasping these updates is essential for maintaining compliance and ensuring product safety. This shift not only underscores a more integrated approach to quality management but also aligns U.S. regulations with international standards, particularly ISO 13485:2016.
Key changes have been highlighted throughout the article, including:
Manufacturers are urged to proactively prepare for these upcoming changes by conducting internal audits, revising control systems, and ensuring that staff are adequately trained on the new requirements. It is vital to emphasize the importance of robust documentation and a comprehensive understanding of the QMSR for successfully navigating this transition.
Ultimately, the shift to QMSR presents an opportunity for manufacturers to enhance their operational efficiency and compliance posture within an increasingly complex regulatory environment. By prioritizing early preparation and adopting a proactive approach to these changes, organizations can not only meet the new standards but also position themselves for success in the global marketplace. Embracing these updates will cultivate a culture of continuous improvement and operational excellence, ensuring that products not only comply with FDA regulations but also meet the highest standards of quality and safety.
What is Bioaccess and how does it assist companies with FDA QSR changes?
Bioaccess delivers tailored solutions to help companies navigate the new FDA Quality System Regulation (QSR) requirements, leveraging over 15 years of experience to ensure prompt adherence and minimize delays in product development and market entry.
What are the key changes in the FDA QSR that will take effect in 2026?
The FDA's Quality Management System Regulation (QMSR) will replace the existing FDA QSR on February 2, 2026. This change aligns closely with ISO 13485:2016 and aims to streamline compliance processes, enhance product safety and efficacy, and increase scrutiny in areas like risk oversight and documentation practices.
What is the transition period for manufacturers to comply with the QMSR?
Manufacturers have a two-year transition period to comply with the QMSR, starting from February 2024.
How does the QMSR differ from the current FDA QSR?
The QMSR introduces an increased focus on risk oversight throughout the product lifecycle and integrates Design for Excellence principles, which aim to embed quality into products from the design stage.
What steps can manufacturers take to prepare for the QMSR changes?
Manufacturers can engage in proactive training and conduct gap analysis to identify necessary adjustments to their control systems in preparation for the QMSR changes.
Does obtaining ISO 13485:2016 certification exempt manufacturers from FDA inspections?
No, while ISO 13485:2016 certification is beneficial, it does not exempt manufacturers from FDA inspections. Thorough adherence to the QMSR is still required.
What is the significance of aligning FDA QSR with ISO 13485:2016 for manufacturers?
The alignment represents a commitment to a more structured approach to quality assurance, streamlining compliance and enhancing the credibility of products in global markets.
Why is it important for companies to revise their assurance systems?
Companies need to revise their assurance systems to reflect the changes in both FDA QSR and ISO standards, ensuring full compliance and maintaining high standards of oversight.