


The article titled "8 Key Insights on FDA 21 CFR Part 820 for Medtech Innovators" serves to provide essential information for Medtech innovators concerning compliance with FDA regulations. It underscores that adherence to FDA 21 CFR Part 820 is vital not only for regulatory compliance but also for ensuring product quality and patient safety. This assertion is reinforced by the article's insights into the importance of quality management systems and the implications of the forthcoming Quality Management System Regulation (QMSR).
The landscape of medical device regulation is evolving, with FDA 21 CFR Part 820 serving as a critical framework governing the quality and safety of products entering the market. For Medtech innovators, understanding this regulation transcends mere compliance; it represents a strategic advantage that can accelerate product development and enhance market readiness. As the industry adapts to new inspection processes and harmonization efforts with international standards like ISO 13485, a pressing question emerges: how can companies effectively navigate these complexities to ensure both compliance and innovation? This article explores eight key insights that illuminate the path forward for Medtech firms striving to thrive in a competitive regulatory environment.
bioaccess® specializes in accelerating compliance with FDA 21 CFR Part 820 by utilizing extensive knowledge of regulatory frameworks and speeding up ethical approvals. Our comprehensive services encompass:
This empowers Medtech innovators to navigate the complexities of FDA 21 CFR Part 820 with agility. With operational efficiencies across Latin America, the Balkans, and Australia, bioaccess® ensures that companies meet regulatory requirements in a fraction of the time compared to conventional markets. This capability is crucial for those seeking to bring innovative medical devices to market swiftly and efficiently, bolstered by our expertise in regulatory navigation and customized solutions for Medtech startups.
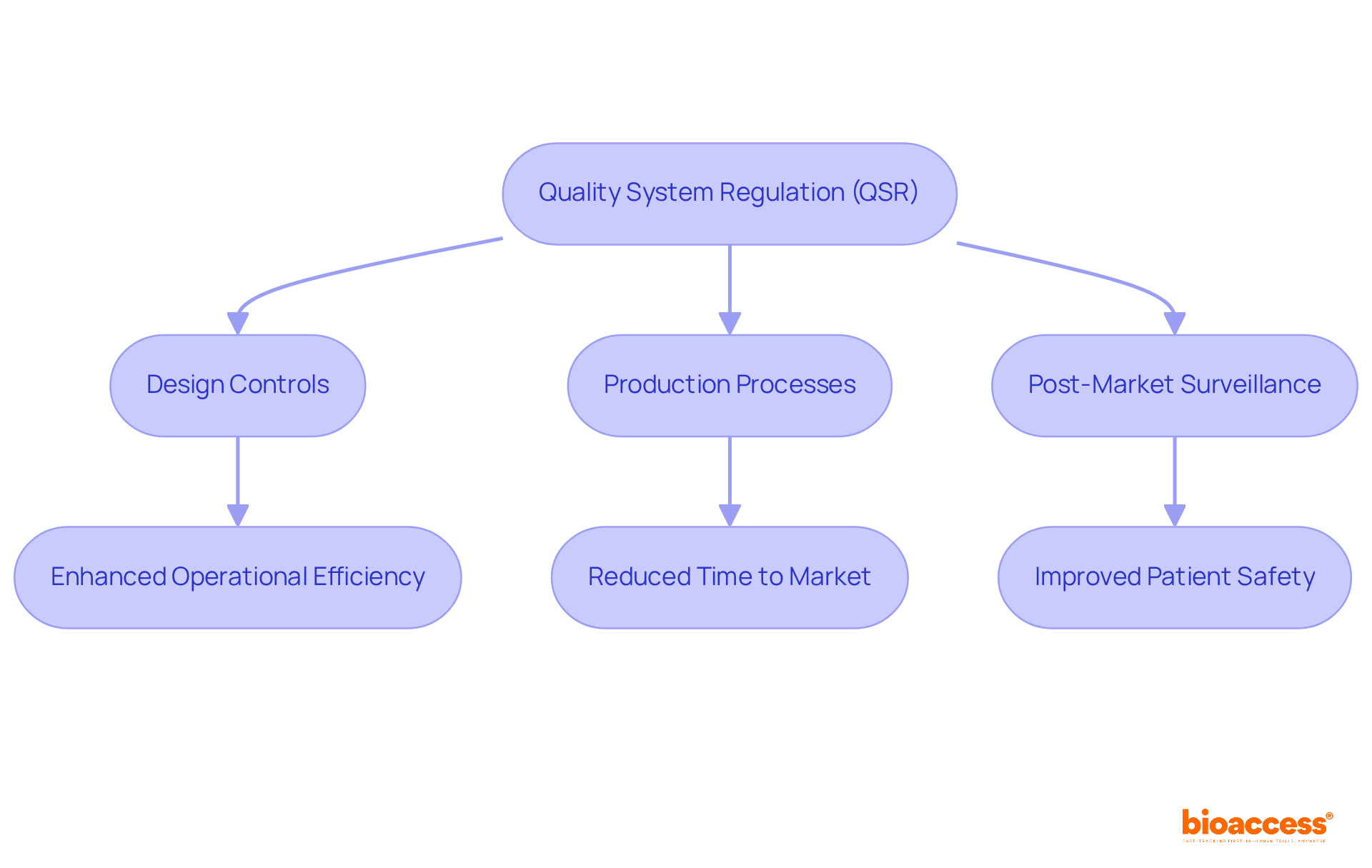
The Quality System Regulation (QSR), which is part of FDA 21 CFR Part 820, serves as the cornerstone for ensuring that medical devices are designed and manufactured to meet stringent safety and effectiveness standards. This regulation encompasses critical components such as design controls, production processes, and post-market surveillance, all essential for maintaining high-quality medical devices. For Medtech innovators, adherence to FDA 21 CFR Part 820 transcends mere regulatory compliance; it is a crucial strategy for ensuring product quality and safeguarding patient safety, which directly impacts market success.
Real-world examples underscore the importance of QSR adherence. Companies that have successfully implemented robust quality management systems (QMS) report enhanced operational efficiency and reduced time to market. For instance, producers that conducted detailed gap analyses and established extensive documentation practices have observed notable improvements in adherence and product quality. These proactive measures not only streamline regulatory processes but also cultivate a culture of quality within organizations.
Current trends reveal a growing emphasis on aligning QSR practices with international standards, such as ISO 13485. This harmonization fosters adherence across various regions and enhances the overall effectiveness of quality management systems. Industry leaders assert that a robust QMS is indispensable for navigating the complexities of medical device regulations, with many advocating for ongoing enhancement and routine audits to identify and address regulatory challenges.
Recent developments in FDA regulations further highlight the significance of adhering to FDA 21 CFR Part 820. The FDA's ongoing efforts to modernize and harmonize its regulations aim to bolster patient safety and improve access to high-quality medical devices. As noted by industry experts, the integration of sex-specific data into the medical device lifecycle represents a significant advancement towards ensuring that products meet diverse patient needs.
Katherine Ruiz, a Regulatory Affairs Expert at bioaccess®, exemplifies the importance of having knowledgeable professionals to navigate these complexities. With her extensive experience advising international producers on securing market approval in Colombia, she underscores the importance of regulatory excellence in ensuring adherence to FDA 21 CFR Part 820. Her background in industrial microbiology and quality management equips her to guide Medtech innovators through the intricacies of regulatory requirements, ultimately enhancing product safety and efficacy.
In summary, the QSR is not merely a regulatory framework; it is a critical element for Medtech innovators striving for excellence in product development and patient care, as outlined in FDA 21 CFR Part 820. By emphasizing QSR adherence and leveraging the expertise of professionals like Katherine Ruiz at bioaccess®, organizations can enhance their market competitiveness while ensuring the safety and effectiveness of their medical devices.
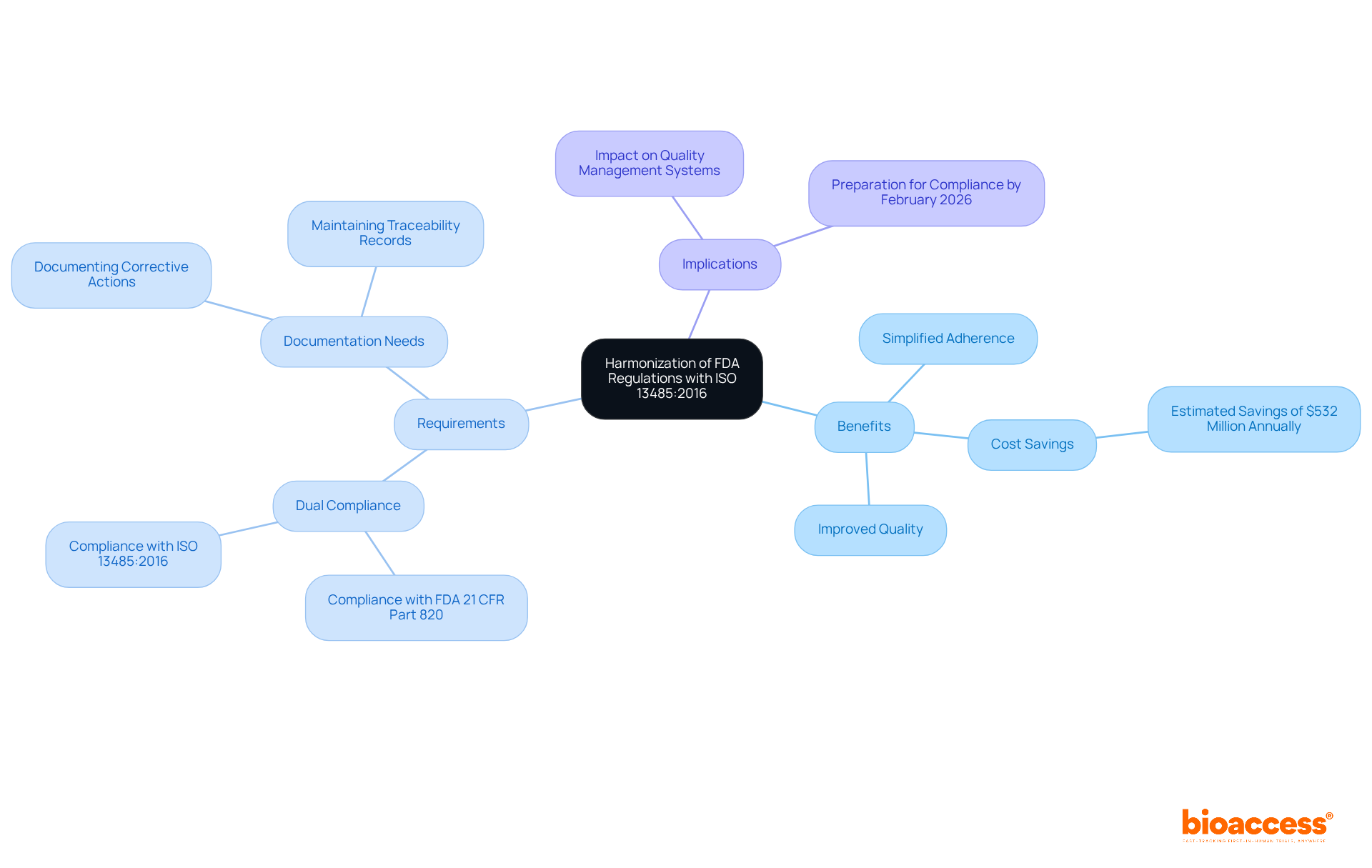
The FDA's recent modifications to FDA 21 CFR Part 820, which include ISO 13485:2016, establish a unified regulatory framework that simplifies adherence for medical device producers. This alignment not only minimizes duplicative efforts but also enhances the overall quality management systems (QMS) of companies. By adopting ISO 13485 standards, Medtech innovators can ensure that their products satisfy both FDA requirements and international quality benchmarks, thereby facilitating smoother entry into global markets.
However, it is crucial to note that the FDA will not accept ISO 13485 certification as a substitute for FDA inspections, underscoring the necessity to comply with both FDA 21 CFR Part 820 and ISO standards. The integration of ISO 13485:2016 is projected to yield significant cost savings, estimated at approximately $532 million annually, while also improving the overall quality of medical devices available to patients.
As the QMSR becomes effective on February 2, 2026, manufacturers must prepare for compliance with FDA 21 CFR Part 820, which includes documenting corrective actions and understanding the challenges introduced by the new regulation.
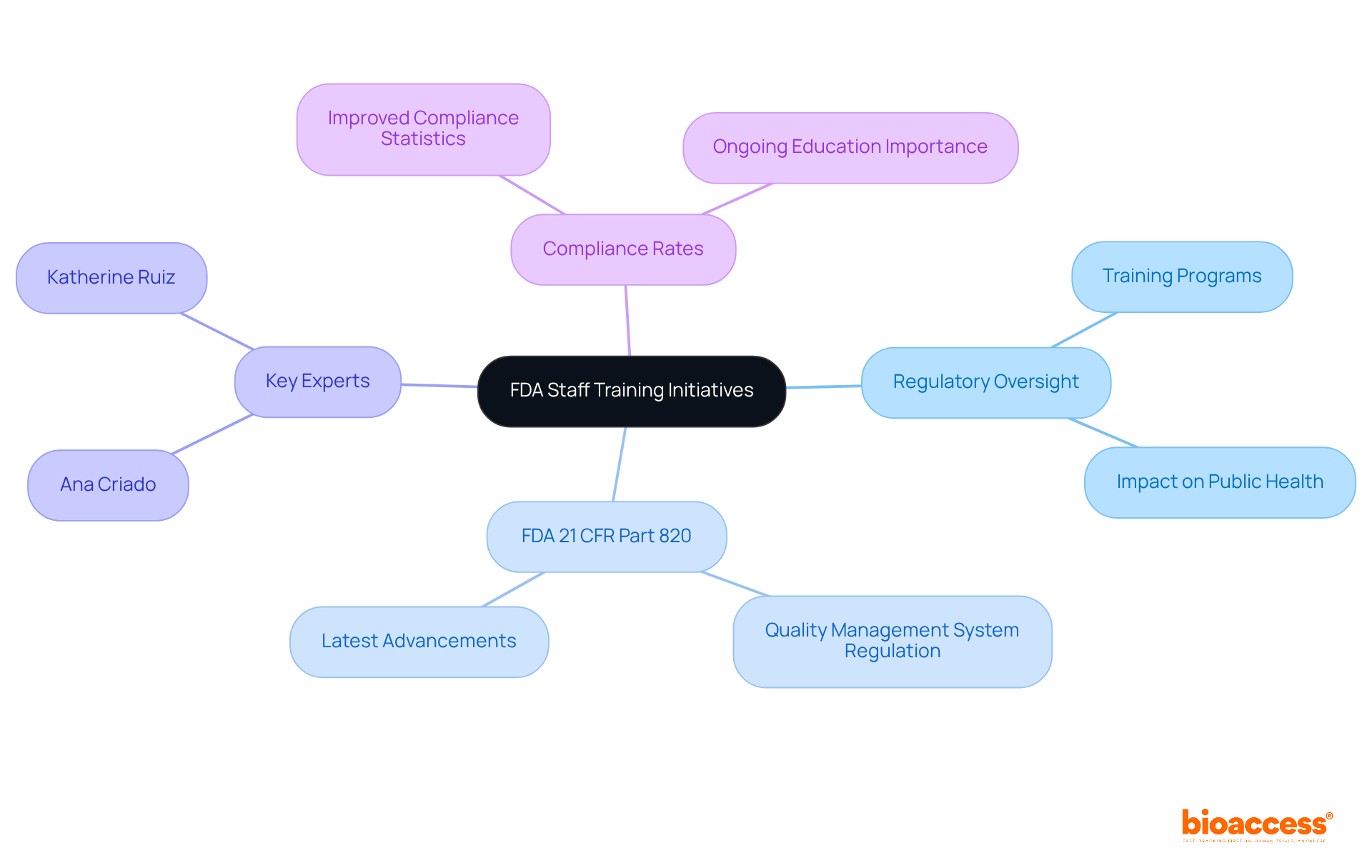
The FDA has launched a series of training initiatives aimed at enhancing the regulatory oversight capabilities of its personnel. These programs emphasize the latest advancements in medical device regulations, particularly the FDA 21 CFR Part 820 regarding Quality Management System Regulation (QMSR). By equipping FDA personnel with up-to-date knowledge and skills related to FDA 21 CFR Part 820, the agency ensures the maintenance of strict regulatory standards and the efficient oversight of the medical device industry, ultimately protecting public health.
Experts in regulatory affairs, such as Ana Criado and Katherine Ruiz, play pivotal roles in this landscape. Recent statistics reveal that enhanced training has resulted in improved compliance rates among manufacturers, underscoring the essential role of ongoing education in sustaining regulatory integrity. Furthermore, the FDA's commitment to continuous professional development illustrates its proactive approach to adapting to the ever-evolving landscape of medical device regulations.
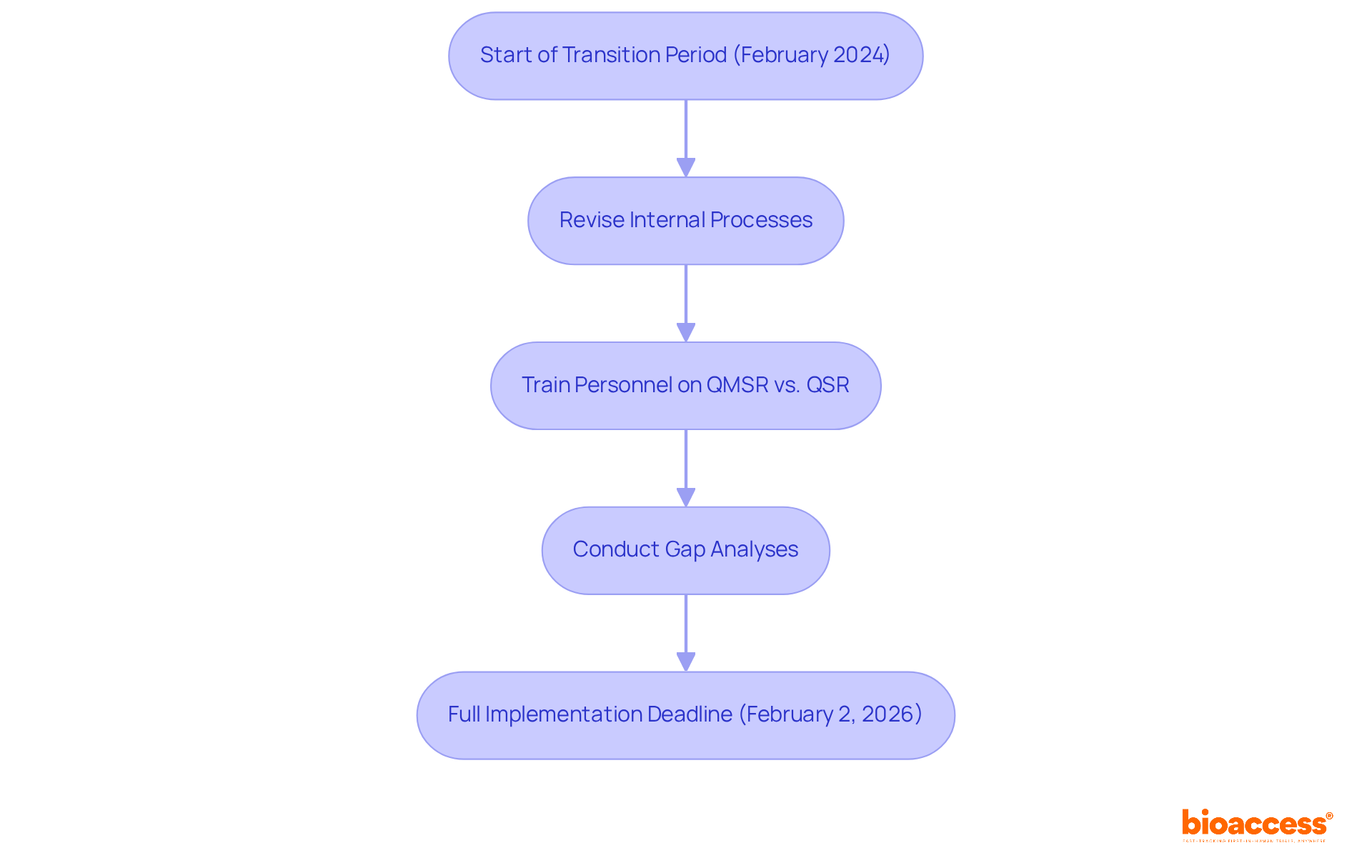
The Quality Management System Regulation (QMSR) is set for full implementation by February 2, 2026, representing a pivotal change for medical device manufacturers. This two-year transition period necessitates immediate action from organizations to align their quality management systems with the new requirements. Key preparation steps include:
Industry experts, such as Ana Criado, Director of Regulatory Affairs and CEO of Mahu Pharma, stress the importance of proactive measures; a recent survey revealed that only 30% of Medtech firms feel adequately prepared for the impending changes. Engaging with the FDA and industry associations during this transition will be vital for ensuring compliance and sustaining market access. As the deadline approaches, organizations must prioritize these preparations to avert disruptions and enhance their operational efficiency under the new regulatory framework. Ana's extensive experience in regulatory affairs, particularly in biomedical engineering and health economics, positions her as a crucial resource for effectively navigating these changes, especially given her consulting work with global companies like General Electric and Omron Healthcare.
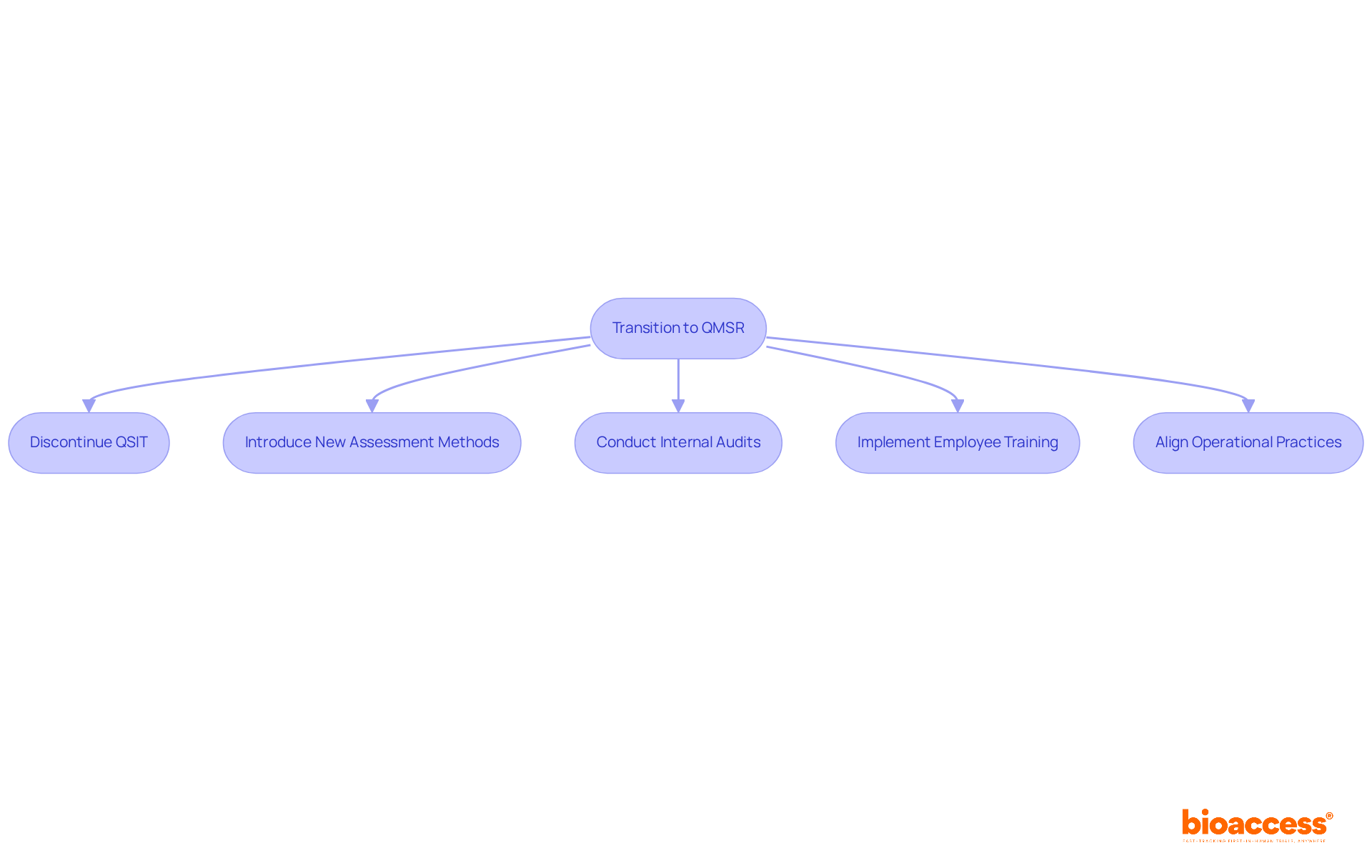
The FDA's transition to the Quality Management System Regulation (QMSR) marks a pivotal shift in evaluation methodologies, underscoring a comprehensive and risk-based approach. This transition involves the discontinuation of the Quality System Inspection Technique (QSIT) and the introduction of new assessment methods that not only evaluate compliance with regulations but also assess the overall effectiveness of quality management systems.
Regulatory specialists note that this development aims to enhance product safety and effectiveness, compelling Medtech firms to prepare for more rigorous evaluations. Data reveals that organizations that proactively adapt to these revised methodologies can significantly improve their compliance rates. For instance, organizations that have undertaken thorough internal audits and employee training have reported heightened readiness for FDA evaluations.
As the industry adapts to these changes, specific case studies of Medtech firms successfully navigating the new landscape highlight the critical need to align operational practices with the FDA's expectations. By prioritizing risk management and robust documentation practices, manufacturers can strategically position themselves within the evolving regulatory environment.
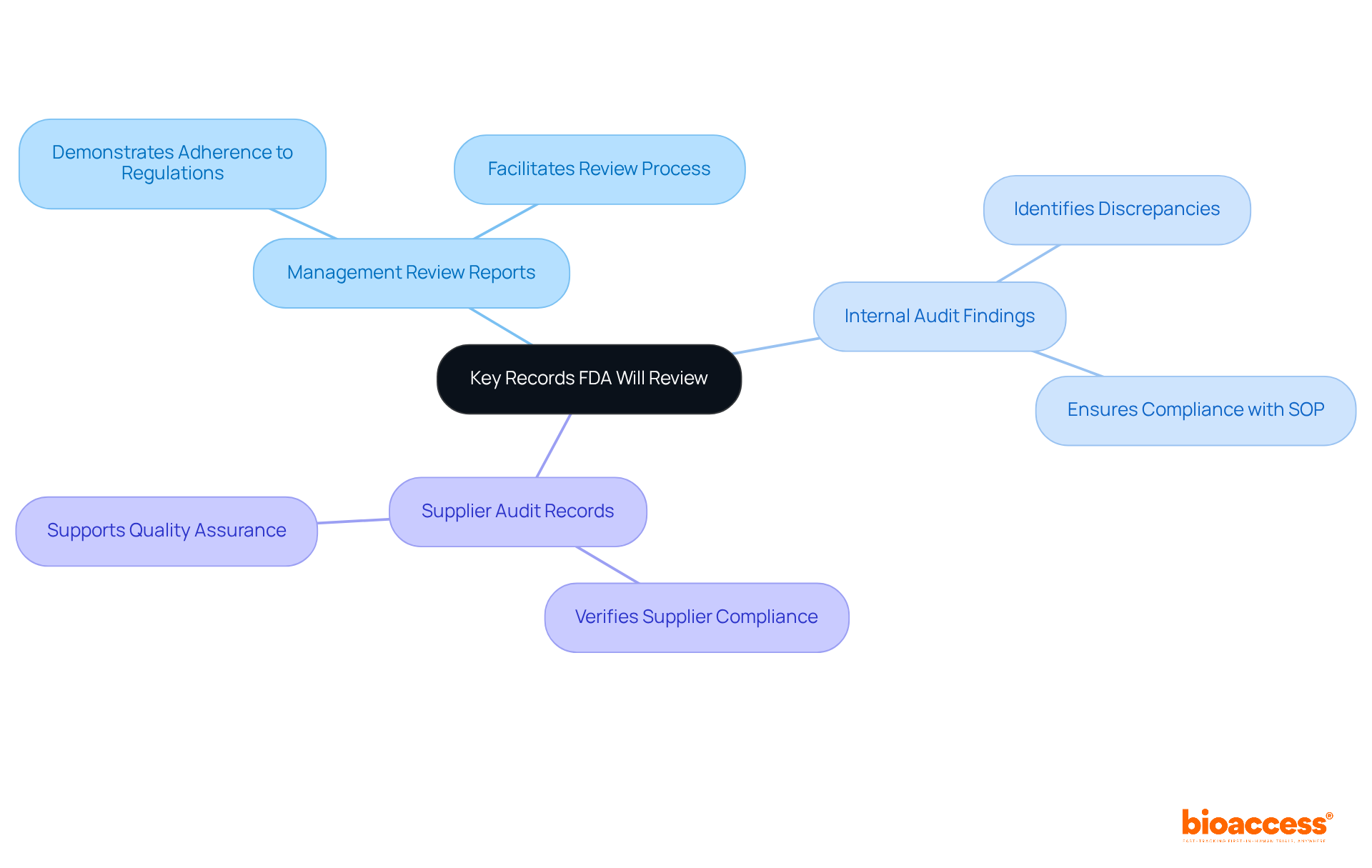
During FDA evaluations, several critical records undergo thorough review, including:
These documents are vital in demonstrating adherence to FDA 21 CFR Part 820 regulations. Additionally, documentation related to design controls, production processes, and post-market surveillance is scrutinized to ensure compliance with established quality system regulations.
Medtech firms must emphasize the thoroughness and precision of these records, as they are crucial for facilitating a seamless review process. For instance, a case study on bioanalytical audit method validation underscores the importance of aligning method validation results with actual sample analysis practices. Discrepancies in storage durations were identified, highlighting the necessity of adhering to standard operating procedures (SOP) to maintain compliance.
Moreover, the audit of data acquisition parameters revealed that proper documentation of analytical processes, as required by FDA 21 CFR Part 820, is essential for achieving accurate results. This underscores the need for meticulous record-keeping practices that not only meet the standards of FDA 21 CFR Part 820 but also enhance the overall quality assurance framework within the organization. By ensuring that all records are easily accessible and well-maintained, Medtech firms can navigate FDA evaluations with greater confidence and efficiency.
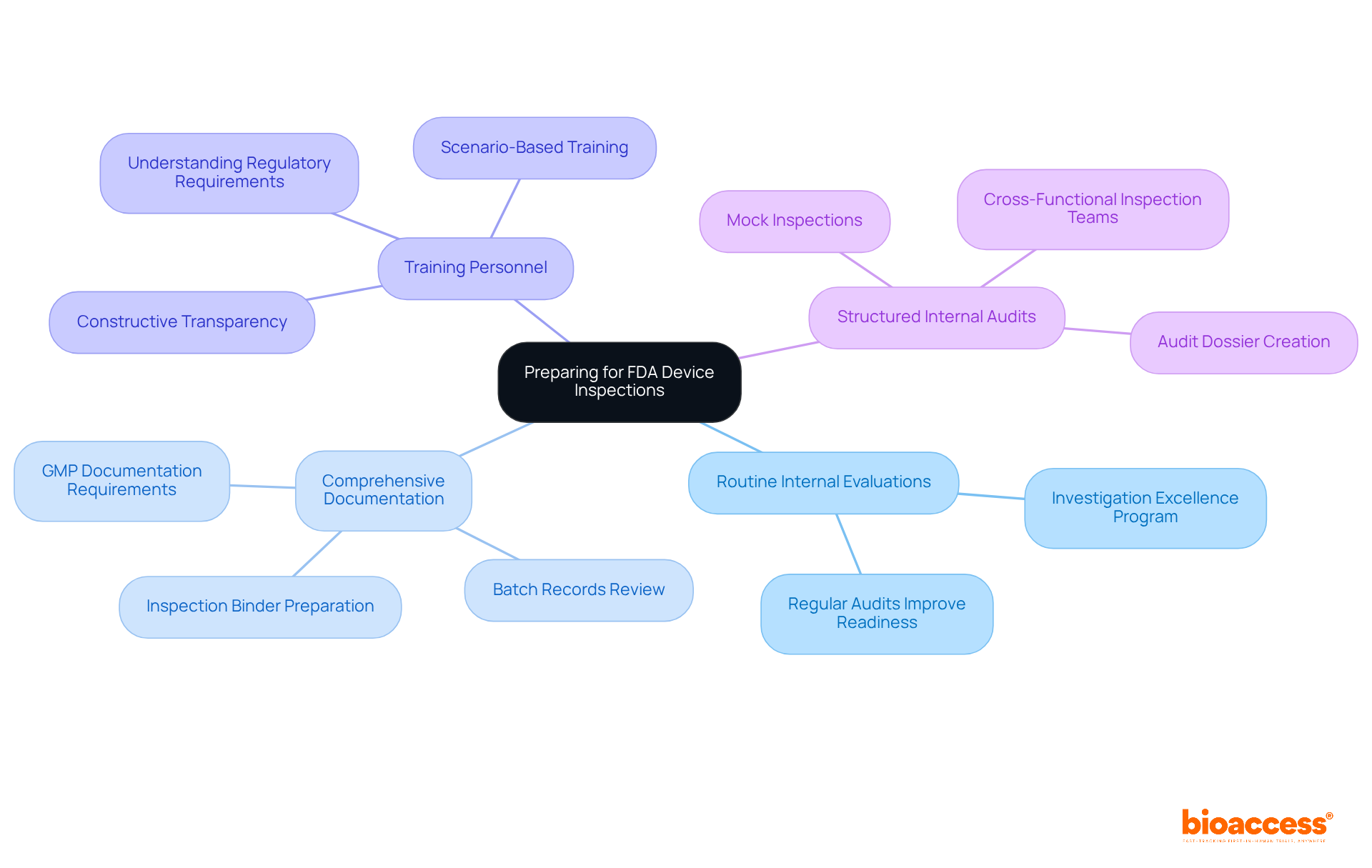
To effectively prepare for evaluations under FDA 21 CFR Part 820, Medtech companies must adopt several best practices. Routine internal evaluations are essential for recognizing and addressing potential regulatory concerns, as they significantly enhance outcomes in accordance with FDA 21 CFR Part 820. For instance, organizations that conduct regular audits often report improved readiness; one biologics manufacturer even reduced their investigation backlog by 80% after implementing an 'Investigation Excellence Program.'
Maintaining comprehensive documentation of all quality processes is vital. The FDA 21 CFR Part 820 emphasizes that all GMP-related documentation must be complete, accurate, and traceable, with inspectors reviewing various records to ensure compliance. This includes ensuring that all completed batch records are reviewed and closed within 30 days of manufacture. Furthermore, gathering product complaints and CAPAs since the previous evaluation is a common request from investigators, underscoring the importance of meticulous preparation.
Training personnel on regulatory requirements is another critical component. Employees should be well-versed in evaluation protocols and understand which inquiries to address during assessments. This training fosters a culture of quality and compliance, encouraging open communication and proactive problem-solving. As industry leaders note, "a truly inspection-ready site operates under the assumption that an FDA investigator could arrive at any time, adhering to the standards outlined in FDA 21 CFR Part 820."
Moreover, organizations should adopt a structured approach to internal audits, as these audits not only help identify gaps but also demonstrate a commitment to continuous improvement. Companies should also prepare for a closeout meeting at the end of an FDA review to discuss observations documented on FDA Form 483. By integrating these strategies into their operational culture, Medtech firms can enhance their readiness for assessments and ensure compliance with FDA 21 CFR Part 820.
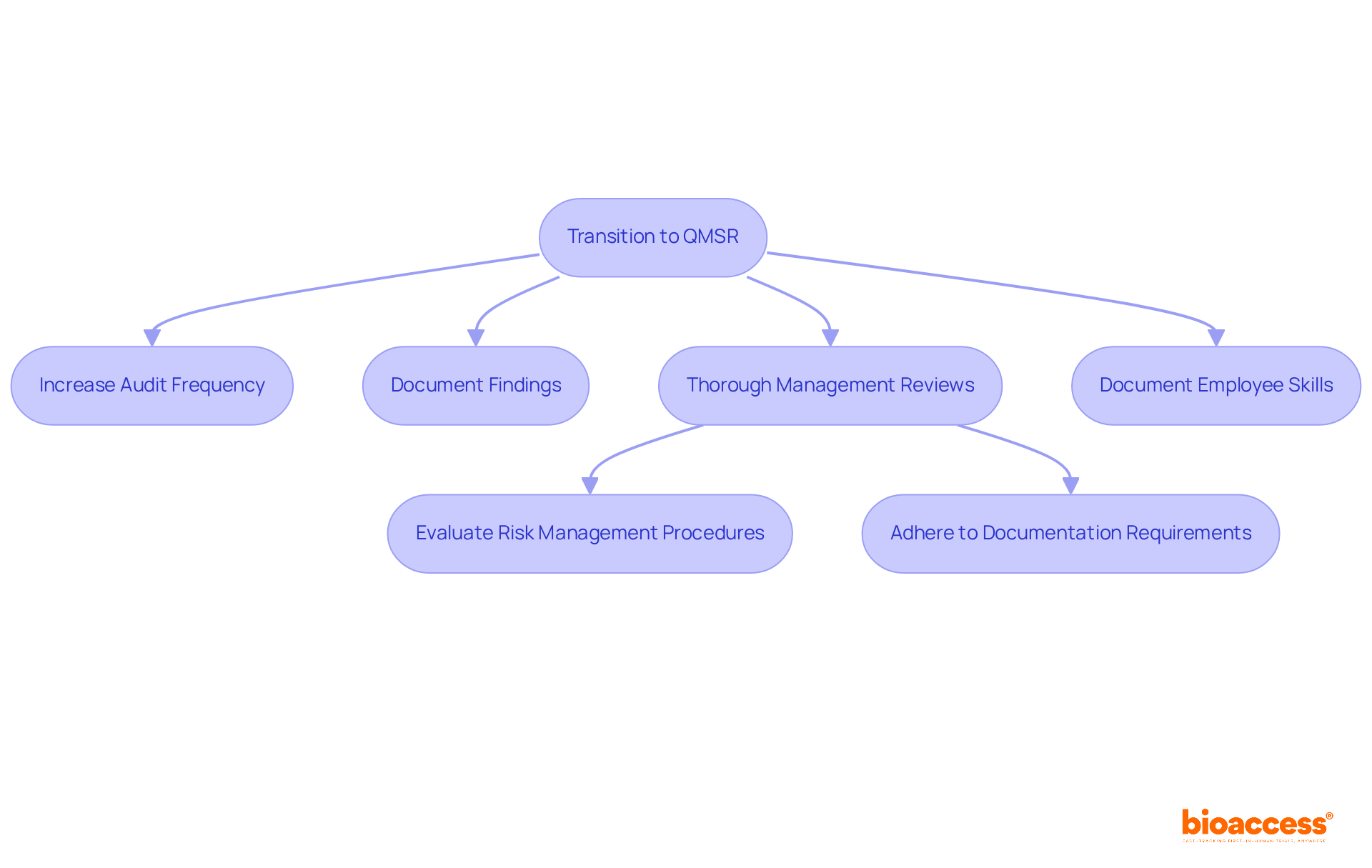
The transition to the Quality Management System Regulation (QMSR) will profoundly impact internal audits and management reviews within Medtech firms. To comply with this new regulatory framework, organizations must embrace a more rigorous approach to these processes. This entails:
Enhanced management reviews should encompass a comprehensive evaluation of:
These elements are crucial for successful FDA inspections. Furthermore, organizations must document employee skills and training as an integral part of their quality system, which is a vital component of the QMSR. The impending compliance deadline of February 2, 2026, underscores the urgency for Medtech firms to refine these processes.
Regulatory specialists emphasize that a well-structured management review process can lead to improved outcomes during FDA evaluations, demonstrating a commitment to quality and accountability. Notably, management reviews and internal audits will no longer be exempt from evaluation under FDA 21 CFR Part 820, marking a significant shift in how firms should approach these processes.
As the QMSR implementation date approaches, companies that prioritize these enhancements—such as conducting a gap analysis to identify discrepancies between current practices and QMSR standards—will be better positioned to navigate the evolving regulatory landscape and mitigate potential compliance risks.
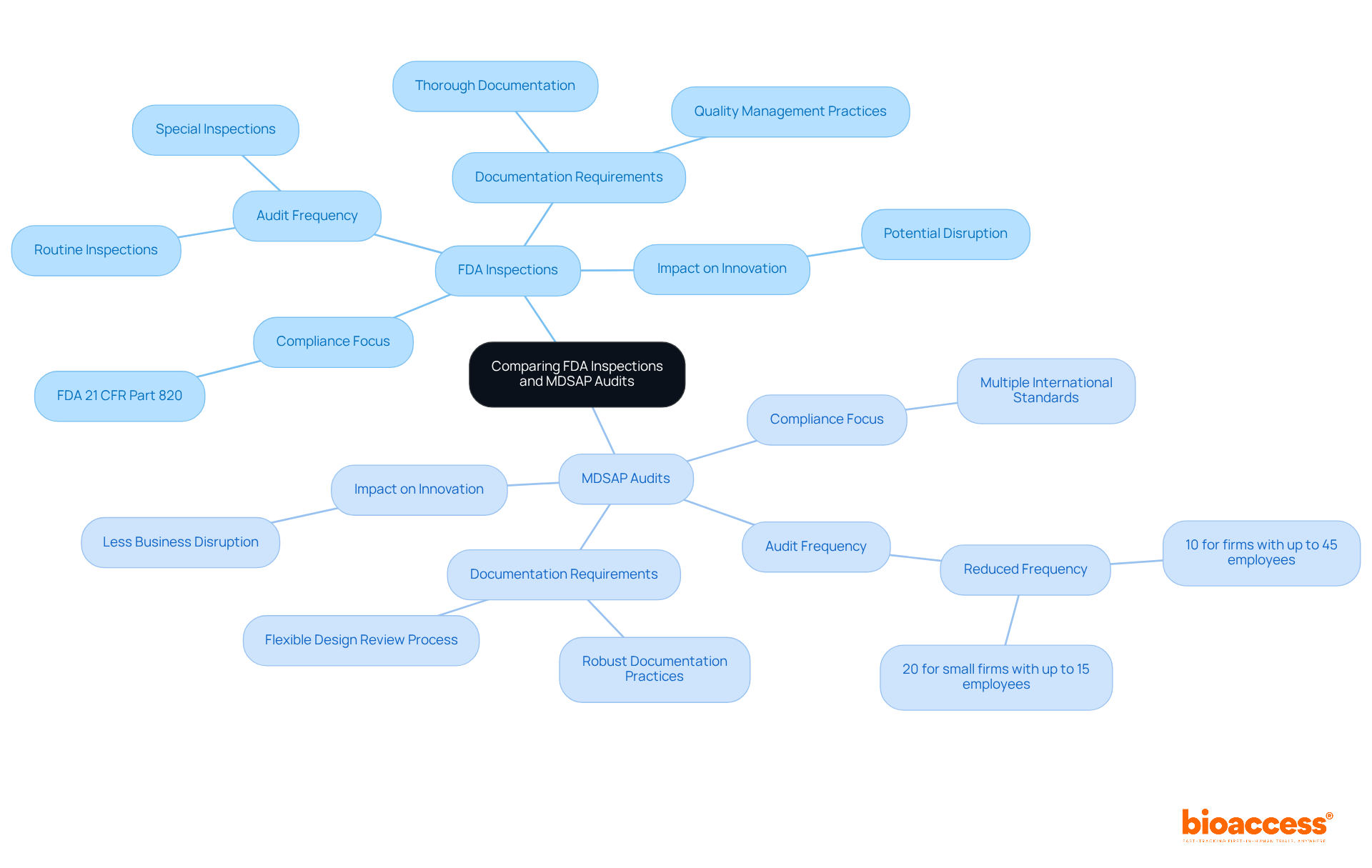
FDA evaluations and MDSAP audits serve comparable functions, albeit through differing methodologies. FDA inspections center on compliance with U.S. regulations, particularly focusing on FDA 21 CFR Part 820. In contrast, MDSAP audits evaluate adherence to multiple international standards within a single audit framework. Companies engaged in MDSAP can experience a reduction in audit frequency—approximately 10% for firms with up to 45 employees and 20% for smaller firms with up to 15 employees—thereby streamlining their operational processes. Nonetheless, they must remain prepared for the stringent standards established by the FDA.
Medtech firms navigating these two systems must maintain vigilance, as MDSAP does not exempt them from the rigorous requirements imposed by FDA 21 CFR Part 820. For example, while MDSAP permits a more flexible design review process, it still demands thorough documentation and strict adherence to quality management practices. Industry professionals have observed that the MDSAP program can significantly decrease the number of audits, resulting in less business disruption and enabling companies to concentrate on innovation and product development.
Experts highlight that transitioning to MDSAP can yield benefits; however, it also introduces complexities due to the integration of various country-specific requirements. This complexity may pose barriers for smaller manufacturers, necessitating careful consideration regarding their participation in MDSAP. Understanding these distinctions is crucial for Medtech innovators aspiring to excel in global markets, ensuring they are well-prepared for both FDA inspections and MDSAP audits in accordance with FDA 21 CFR Part 820. Furthermore, maintaining comprehensive records of complaints for post-market surveillance is essential under both frameworks, underscoring the significance of robust documentation practices.
Navigating the complexities of FDA 21 CFR Part 820 is paramount for Medtech innovators dedicated to ensuring the safety and effectiveness of their medical devices. This regulation, which encompasses the Quality System Regulation (QSR), provides a vital framework that not only facilitates compliance but also enhances product quality and market success. By thoroughly understanding and adhering to these regulations, organizations can strategically position themselves in a competitive landscape.
The article underscores several critical insights, including:
Engaging with experts and leveraging resources like bioaccess® can significantly streamline this journey.
Ultimately, the implications of FDA 21 CFR Part 820 transcend mere compliance; they embody a commitment to quality and patient safety that can catalyze innovation and operational excellence within the Medtech industry. As the regulatory landscape evolves, embracing these insights and preparing for forthcoming changes will empower organizations to navigate challenges effectively and enhance their competitive edge. Taking decisive action now will not only ensure compliance but also cultivate a culture of continuous improvement that benefits both manufacturers and patients alike.
What is bioaccess® and what services do they provide?
bioaccess® specializes in accelerating compliance with FDA 21 CFR Part 820 by offering services such as feasibility assessments, investigator selection, and meticulous project management, helping Medtech innovators navigate regulatory complexities efficiently.
Why is FDA 21 CFR Part 820 important for Medtech innovators?
FDA 21 CFR Part 820 is crucial for ensuring that medical devices are designed and manufactured to meet safety and effectiveness standards. Adherence to this regulation is essential for maintaining product quality and safeguarding patient safety, which directly impacts market success.
What are the critical components of the Quality System Regulation (QSR)?
The QSR includes design controls, production processes, and post-market surveillance, all of which are essential for maintaining high-quality medical devices.
How can companies benefit from adhering to QSR?
Companies that implement robust quality management systems (QMS) report enhanced operational efficiency and reduced time to market. Proactive measures like gap analyses and extensive documentation practices improve adherence and product quality.
What is the significance of aligning QSR practices with international standards like ISO 13485?
Aligning QSR practices with ISO 13485 fosters adherence across various regions and enhances the overall effectiveness of quality management systems, facilitating smoother regulatory compliance.
What recent developments in FDA regulations are noteworthy?
The FDA is modernizing and harmonizing its regulations to bolster patient safety and improve access to high-quality medical devices. Notably, the integration of sex-specific data into the medical device lifecycle is a significant advancement.
What role do professionals like Katherine Ruiz play in regulatory compliance?
Katherine Ruiz, a Regulatory Affairs Expert at bioaccess®, emphasizes the importance of knowledgeable professionals in navigating regulatory complexities, ensuring adherence to FDA 21 CFR Part 820, and enhancing product safety and efficacy.
What changes have been made to FDA 21 CFR Part 820 regarding ISO 13485:2016?
Recent modifications to FDA 21 CFR Part 820 have established a unified regulatory framework that simplifies adherence for medical device producers, minimizing duplicative efforts and enhancing quality management systems.
Will ISO 13485 certification replace FDA inspections?
No, the FDA will not accept ISO 13485 certification as a substitute for FDA inspections, so compliance with both FDA 21 CFR Part 820 and ISO standards is necessary.
What is the projected impact of the integration of ISO 13485:2016?
The integration is expected to yield significant cost savings, estimated at approximately $532 million annually, while improving the overall quality of medical devices available to patients.
When does the Quality Management System Regulation (QMSR) become effective?
The QMSR will become effective on February 2, 2026, and manufacturers must prepare for compliance with FDA 21 CFR Part 820, including the documentation of corrective actions.