


The article titled "8 Key Insights on Labeling FDA Guidance for Manufacturers" presents critical insights into the implications of FDA labeling guidance for manufacturers operating within the medical device industry.
It underscores the necessity of understanding and adhering to these guidelines, as this compliance is vital for enhancing product credibility and driving commercial success.
Manufacturers who align their practices with FDA requirements not only improve patient safety but also bolster their market competitiveness.
This alignment ultimately positions them for greater success in a challenging landscape.
Navigating the intricate landscape of FDA labeling guidance is essential for manufacturers aiming to ensure compliance and enhance their market presence. As the regulatory environment evolves, understanding the implications of these guidelines can unlock significant advantages in product development and marketing strategies. However, with the introduction of new requirements and the urgency to adapt, how can companies effectively align their practices to not only meet compliance but also leverage it for commercial success?
bioaccess® plays a pivotal role in guiding producers through the complexities of FDA compliance, particularly in relation to labeling FDA guidance, with its comprehensive acceleration services for clinical trials. With over 50 pre-qualified sites activated in less than eight weeks, bioaccess® leverages its extensive expertise in regulatory requirements to expedite ethical approvals, enabling producers to efficiently comply with labeling FDA guidance. This approach not only accelerates the time to market but also enhances the overall quality of the tagging process.
By delivering FDA/EMA/MDR-ready datasets, facilitating subject recruitment, and providing trial data, bioaccess® empowers manufacturers to concentrate on innovation while ensuring adherence to labeling FDA guidance in LATAM, Eastern Europe, and Australia.
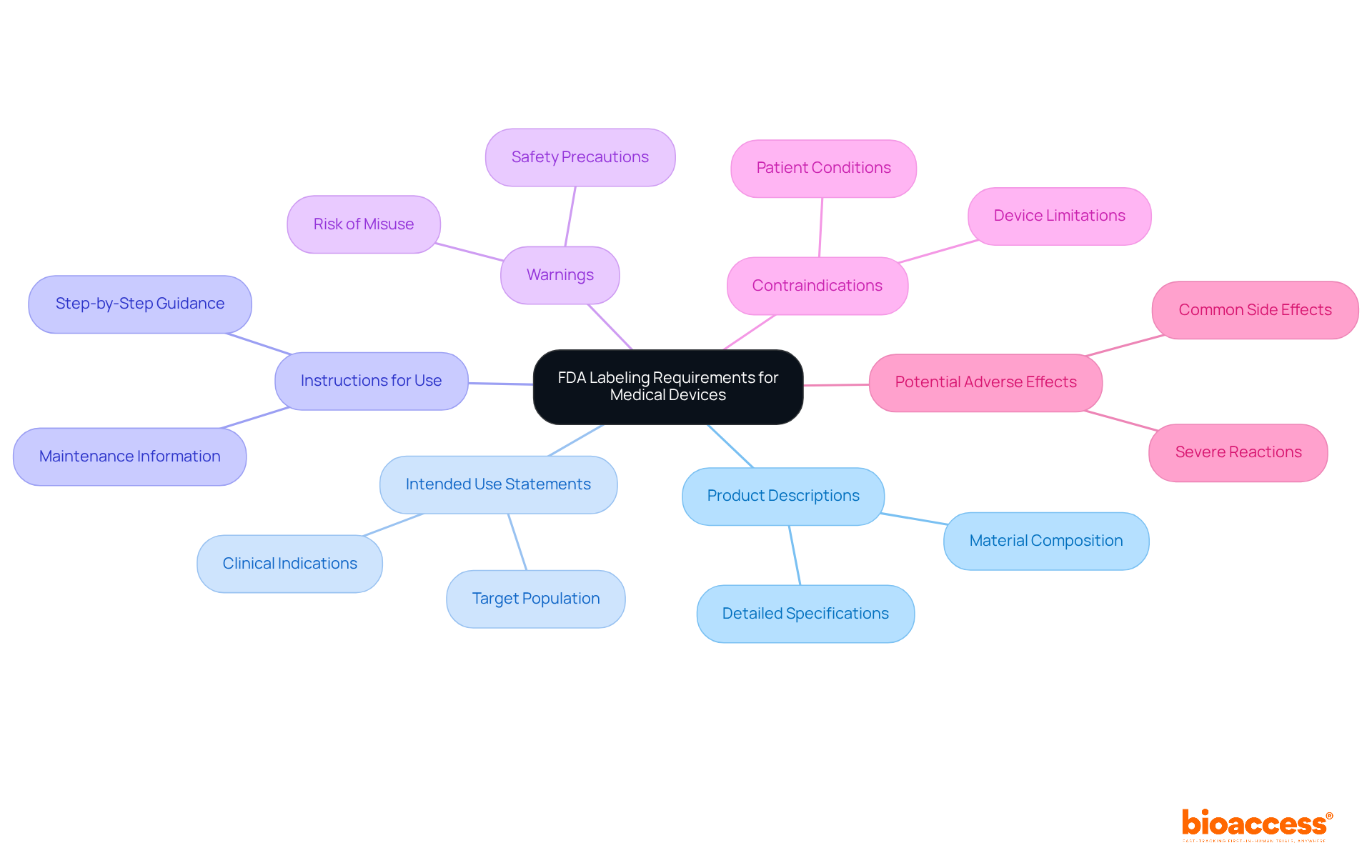
Key FDA requirements for medical devices encompass precise product descriptions, intended use statements, and clear instructions for use. Manufacturers are also obligated to include warnings, contraindications, and potential adverse effects. Adhering to these standards is crucial; it guarantees that the markings are not deceptive and provides sufficient information for the safe and effective use of the device.
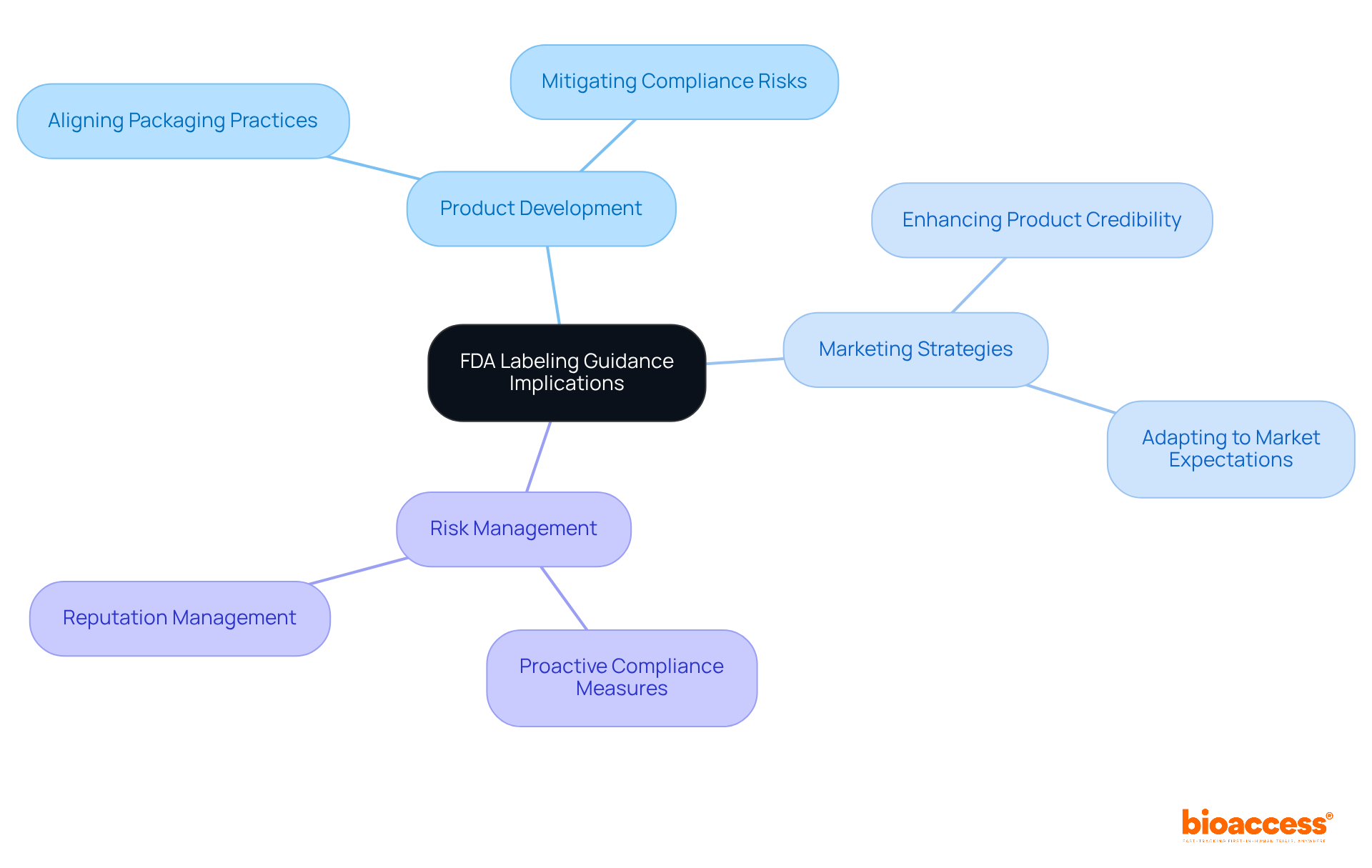
The implications of labeling FDA guidance on product information are profound, extending well beyond mere compliance; they play a pivotal role in shaping product development, marketing strategies, and risk management. Experts, including Ana Criado, Director of Regulatory Affairs and a professor in biomedical engineering, underscore the necessity for producers to stay updated on guidance changes.
With her extensive background in regulatory affairs and health economics, Ana asserts that aligning packaging practices with current expectations is not just beneficial, but essential. This proactive approach not only mitigates risks associated with non-compliance but also enhances the product's credibility in the market.
Her consultancy work with global companies, along with her leadership at Mahu Pharma, illustrates the tangible benefits of such alignment.
To gain FDA approval, producers must convincingly demonstrate the significant clinical advantages of their devices according to the labeling FDA guidance. This necessitates the provision of robust data from clinical trials that substantiate claims of safety and efficacy.
With bioaccess®, producers can:
By effectively communicating these compelling benefits, manufacturers can significantly enhance their product's appeal to both regulators and healthcare providers, ultimately leading to improved patient outcomes.
Manufacturers can leverage FDA compliance as a fundamental component of their commercial strategy. By ensuring that their labeling complies with labeling FDA guidance, they build trust with healthcare providers and patients. This trust often translates into increased sales and market share, as compliant products are perceived as safer and more reliable. Furthermore, adherence to compliance can facilitate smoother interactions with oversight agencies, thereby enhancing commercial success.
Experts like Ana Criado, Director of Regulatory Affairs and a professor in biomedical engineering, assert that understanding the nuances of labeling FDA guidance is essential for manufacturers. Her extensive experience in oversight matters—particularly in the context of medical devices and cannabis legislation in Colombia—underscores the necessity of having informed professionals steering compliance initiatives. This expertise not only aids in meeting compliance standards but also positions companies advantageously within the competitive landscape.
Manufacturers must be prepared to make strategic adjustments in response to changes in labeling FDA guidance. This necessity underscores the importance of:
By leveraging bioaccess®'s expert services for expedited clinical trials—including regulatory approval, patient recruitment, and trial data management—companies can navigate the complexities of the Latin American Medtech landscape more effectively. Remaining nimble and receptive to guidance updates not only allows producers to uphold compliance but also guarantees that their products remain competitive in the market.
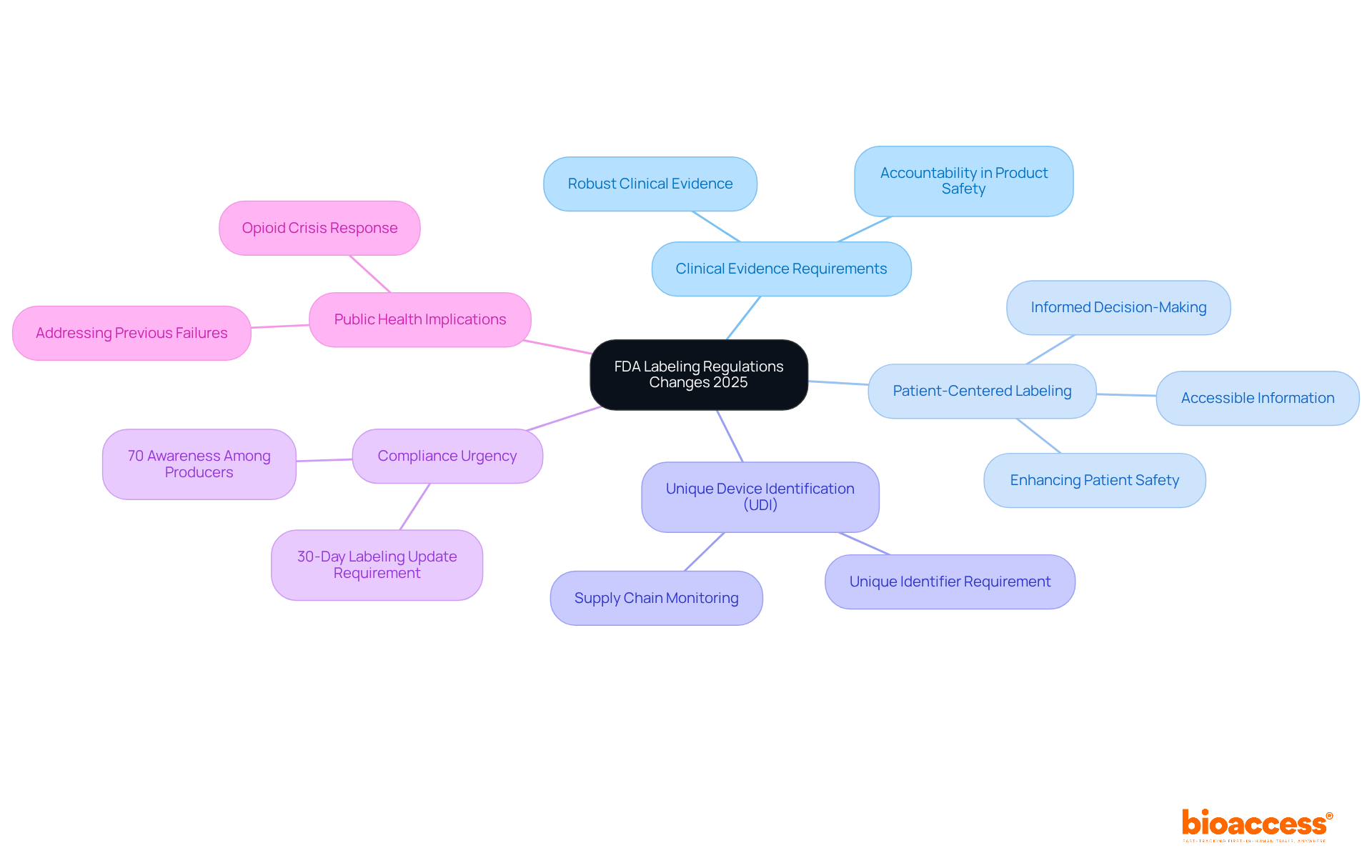
The FDA's revised packaging regulations for 2025 introduce substantial modifications that producers must adapt to in order to remain compliant. Key differences include:
Industry analysts have observed that around 70% of producers are aware of these changes, underscoring the urgency for compliance. As the FDA continues to update its packaging requirements, manufacturers must remain informed and proactive in adapting their practices according to the labeling FDA guidance. This evolution in marking not only seeks to enhance patient safety but also demonstrates the FDA's commitment to addressing previous public health shortcomings, particularly concerning the opioid crisis.
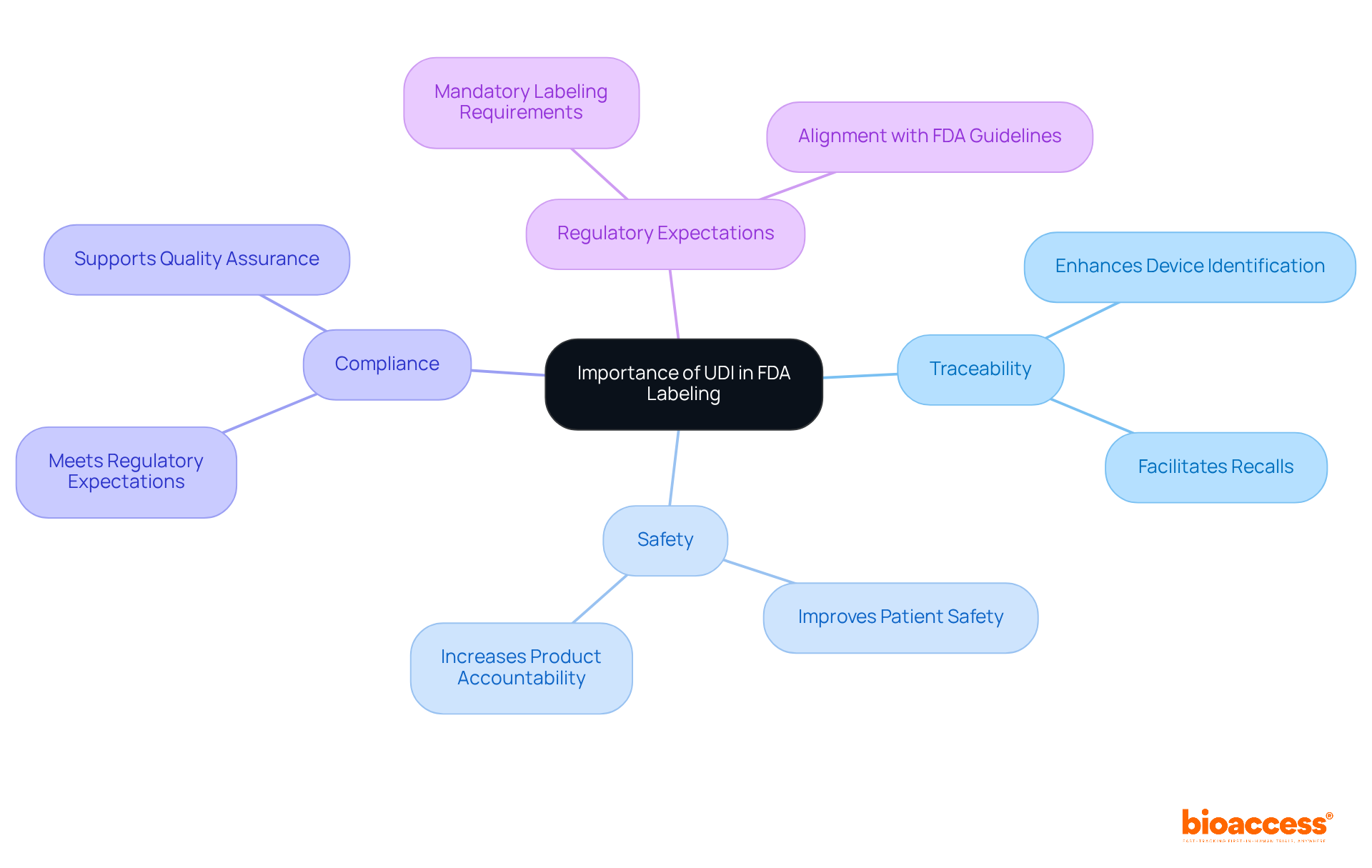
Unique Device Identification (UDI) stands as a crucial component of FDA marking, fundamentally enhancing traceability and safety within the medical device supply chain. In accordance with labeling FDA guidance, manufacturers are mandated to incorporate UDI on their labels, which significantly aids in the identification of devices during recalls or adverse events. Compliance with UDI requirements not only meets regulatory expectations but also bolsters patient safety and product accountability.
Manufacturers must prioritize staying informed about changes in labeling FDA guidance related to product information to ensure ongoing compliance. This can be achieved through:
Experts like Ana Criado, Director of Regulatory Affairs and a professor in biomedical engineering with a degree in chemical pharmacology and a master's in health economics & pharmacoeconomics, emphasize the importance of being proactive in monitoring guidance changes. Her vast background, which includes positions at Colombia’s oversight agency INVIMA and as an advisor for international firms like General Electric, Omron Healthcare, and Mindray, highlights the need for producers to modify their practices accordingly and steer clear of possible hazards linked to outdated markings.
The overall effect of FDA guidance on producers is substantial, influencing product development, marketing strategies, and compliance with regulations. By adhering to FDA guidance, producers can significantly enhance their credibility, improve patient safety, and ultimately drive commercial success. It is essential for manufacturers to grasp the nuances of labeling FDA guidance to navigate the complex regulatory landscape effectively and ensure the successful launch of their products.
Navigating the intricacies of FDA labeling guidance is essential for manufacturers aiming to achieve compliance and success in the competitive medical device market. This comprehensive understanding not only aids in meeting regulatory requirements but also enhances product credibility and patient safety, ultimately leading to improved market performance.
Throughout this article, we have explored key insights, including:
By leveraging services like those offered by bioaccess®, manufacturers can streamline their compliance processes, ensuring they are well-equipped to adapt to evolving guidance while maintaining a focus on innovation and patient outcomes.
As the landscape of FDA labeling continues to evolve, staying informed and proactive is paramount. Manufacturers are encouraged to prioritize ongoing education and adaptation to these regulatory shifts. Embracing this approach not only safeguards compliance but also positions products favorably within the market, fostering trust and enhancing overall commercial success.
What is bioaccess® and how does it assist manufacturers with FDA compliance?
bioaccess® assists manufacturers by guiding them through the complexities of FDA compliance, particularly related to labeling FDA guidance. It offers comprehensive acceleration services for clinical trials, helping producers expedite ethical approvals and efficiently comply with labeling requirements.
How quickly can bioaccess® activate clinical trial sites?
bioaccess® can activate over 50 pre-qualified clinical trial sites in less than eight weeks.
What benefits does bioaccess® provide to manufacturers?
bioaccess® delivers FDA/EMA/MDR-ready datasets, facilitates subject recruitment, and provides trial data, allowing manufacturers to focus on innovation while ensuring adherence to labeling FDA guidance in regions like LATAM, Eastern Europe, and Australia.
What are the key FDA labeling requirements for medical devices?
Key FDA labeling requirements include precise product descriptions, intended use statements, clear instructions for use, warnings, contraindications, and potential adverse effects. Compliance with these standards is crucial for safe and effective device usage.
What are the implications of FDA labeling guidance for manufacturers?
The implications of FDA labeling guidance extend beyond compliance; they significantly influence product development, marketing strategies, and risk management. Staying updated on guidance changes is essential for manufacturers to mitigate risks and enhance product credibility.
Why is it important for manufacturers to align packaging practices with current FDA expectations?
Aligning packaging practices with current FDA expectations is important as it mitigates risks associated with non-compliance and enhances a product's credibility in the market. This proactive approach is essential for successful product development and marketing.