


The article addresses the critical strategies necessary to enhance diversity in clinical trials for underrepresented populations, underscoring the significance of inclusive research practices. It delineates various approaches, including:
All of which are designed to confront historical mistrust and elevate participation rates. These efforts are essential for achieving more effective and equitable healthcare solutions, ultimately reinforcing the need for collaboration in clinical research.
Diversity in clinical trials transcends mere regulatory compliance; it is an essential component that profoundly impacts the efficacy and safety of medical treatments across diverse populations. As the healthcare landscape continues to evolve, the inclusion of underrepresented groups in research studies becomes increasingly critical to ensure that new therapies are effective for everyone.
Nevertheless, a pressing challenge persists: how can the medical research community effectively bridge the gap and encourage participation among these varied populations? This article delves into eight innovative strategies aimed at enhancing diversity in clinical trials, addressing historical mistrust, systemic barriers, and the transformative potential of community engagement alongside digital health technologies.
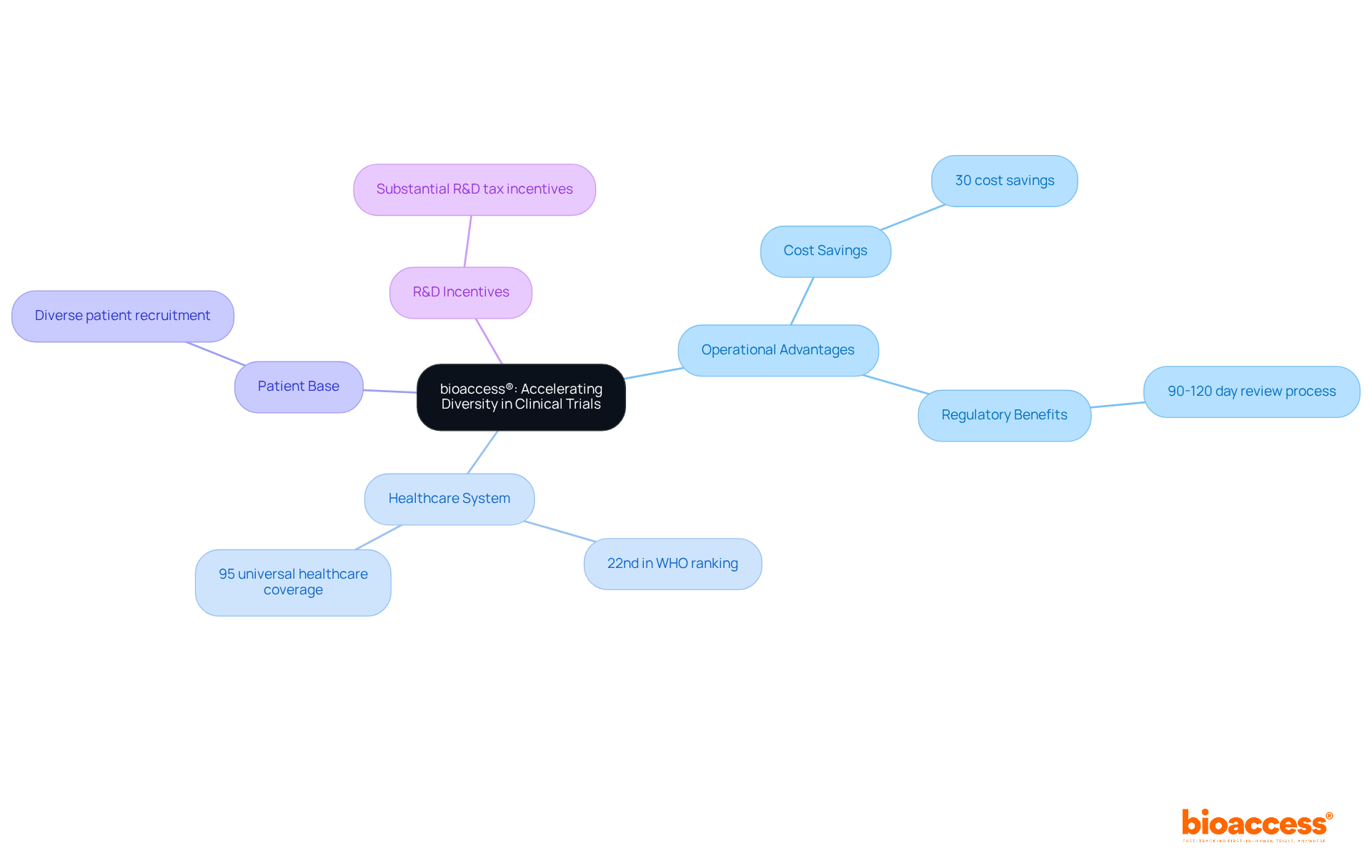
bioaccess® leverages its operational advantages across Latin America, particularly in Colombia, to enhance diversity in research studies. Colombia presents substantial competitive benefits, including cost savings exceeding 30% compared to North America and Western Europe, alongside a regulatory review process that typically spans only 90 to 120 days. The nation's healthcare system is esteemed, ranked 22nd by the World Health Organization, with its hospitals recognized among the finest in Latin America, having successfully undergone a stringent ICH/GCP certification process.
Moreover, with a population surpassing 50 million and approximately 95% covered by universal healthcare, Colombia offers a diverse patient base for recruitment. By capitalizing on these strengths, bioaccess® accelerates the recruitment of underrepresented populations for clinical trials, ensuring that studies accurately reflect the demographics of the populations they aim to serve. This strategy not only hastens the research process but also enriches the data collected, yielding more comprehensive and applicable results in medical research.
Furthermore, investments in science, technology, and innovation initiatives in Colombia benefit from substantial R&D tax incentives, enhancing the overall attractiveness of conducting research in the region.
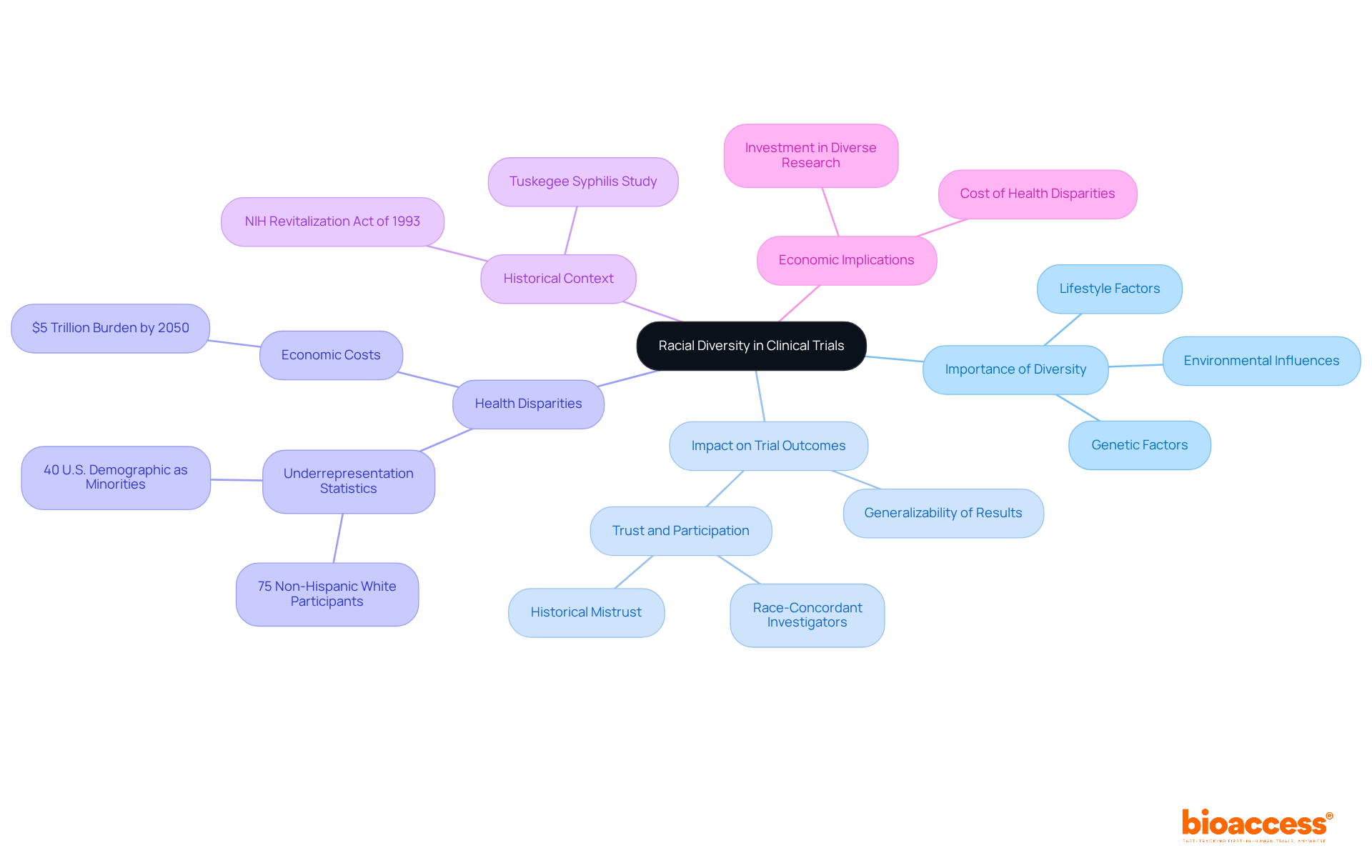
Racial variety in medical studies is crucial for guaranteeing that treatments work effectively across different demographic groups. Research indicates that genetic, environmental, and lifestyle factors significantly influence individual responses to medications. For example, Black and Hispanic individuals are frequently part of underrepresented populations in clinical trials, resulting in a lack of comprehension about how these groups respond to treatments. By including a racially diverse participant pool, researchers can gain insights into these variations, ultimately leading to more tailored and effective healthcare solutions.
The effect of diversity on trial outcomes is profound. Trials that successfully incorporate diverse populations tend to yield results that are more generalizable and applicable to the broader population. For instance, a study discovered that Black patients exhibited a 12.6% rise in interest in participating in research conducted by race-concordant investigators, emphasizing the significance of representation in building trust and involvement.
Moreover, the lack of diversity jeopardizes the validity of study outcomes. A lack of representation in underrepresented populations clinical trials may lead to significant health disparities, as treatments might not be effective for all demographic groups. This is especially troubling considering that racial and ethnic minorities make up 40% of the U.S. demographic, yet 75% of participants in studies that approved 53 new medications in 2020 were non-Hispanic White, underscoring the disparities in underrepresented populations in clinical trials.
Expert views highlight that genetic factors influencing drug effectiveness can differ among demographics, stressing the importance of varied study participants. The historical context of the Tuskegee Syphilis Study has established a legacy of mistrust among African American communities, complicating recruitment efforts. By tackling these inequalities and increasing diversity in research studies, scientists can enhance health equity and ensure that underrepresented populations in clinical trials benefit from new treatments that are effective for all groups. Furthermore, the economic effect of health inequities is considerable, with projections indicating that the absence of representation in medical studies could burden society by more than $5 trillion for diabetes alone by 2050.
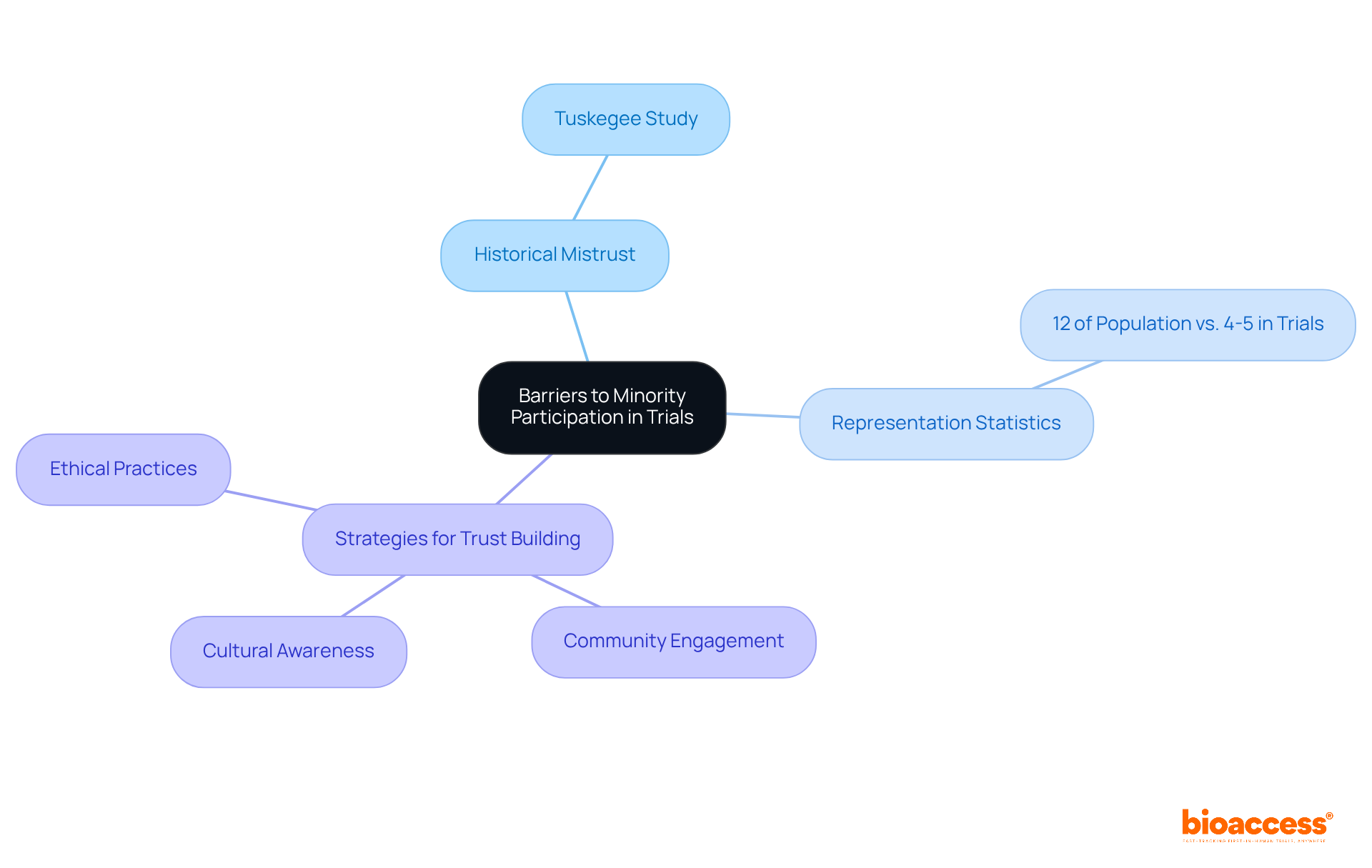
Historical occurrences, particularly the Tuskegee Syphilis Study, have instilled a profound distrust among minority groups towards medical studies. This skepticism is further intensified by ongoing disparities in healthcare access and treatment outcomes.
For instance, although African Americans constitute 12% of the U.S. demographic, they represent only 4 to 5% of research participants, highlighting the issue of underrepresented populations in clinical trials.
To effectively dismantle these barriers, researchers must recognize this historical context and proactively engage in fostering trust through transparent communication and ethical practices.
Community-based participatory research (CBPR) models are essential in this endeavor, cultivating enduring relationships between researchers and minority communities. By implementing strategies that prioritize cultural awareness and respect, medical studies can achieve greater inclusivity and accurately reflect the needs of underrepresented populations in clinical trials.
This dedication to inclusivity not only bolsters the validity of research findings but also guarantees that the advantages of medical advancements are equitably distributed across all demographics.
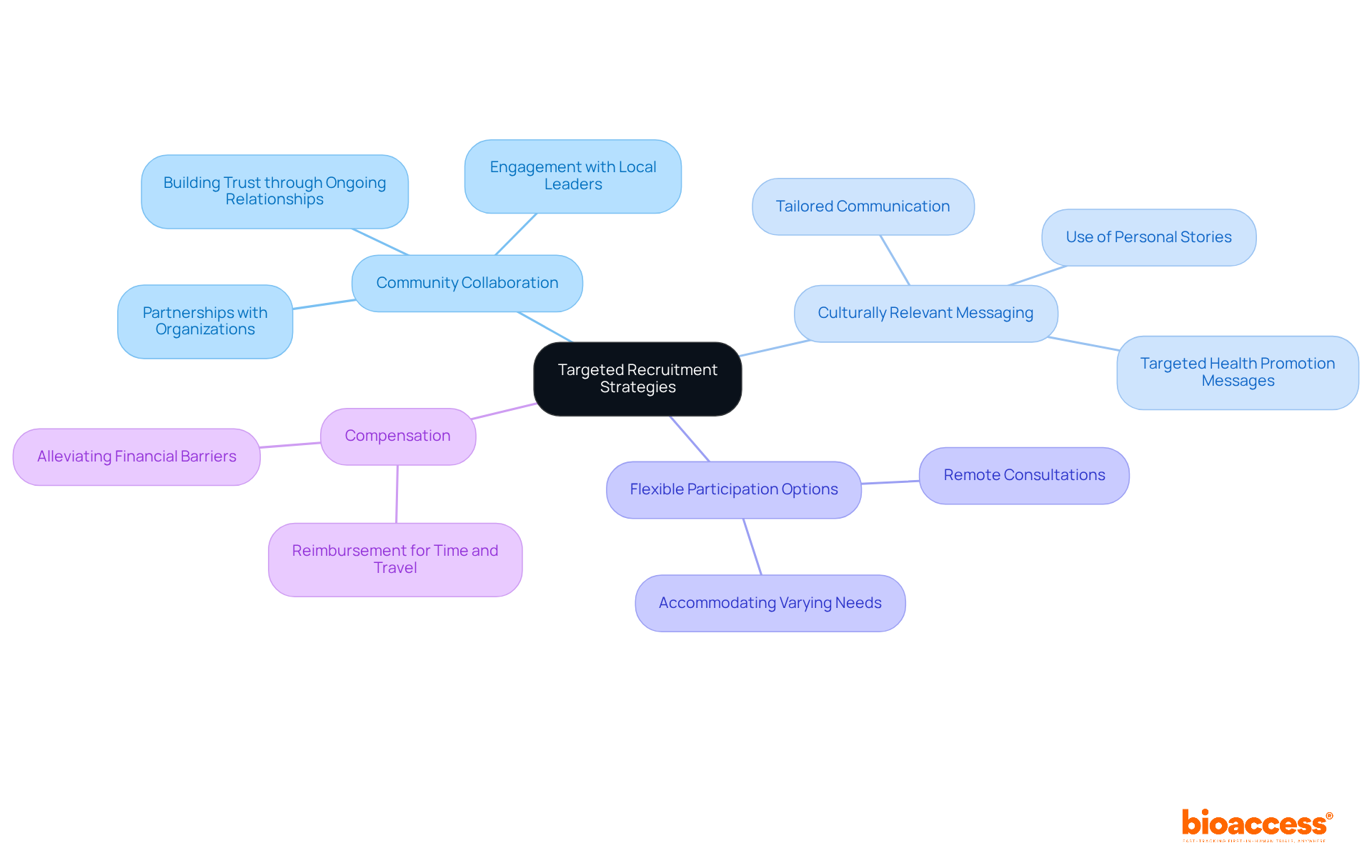
To effectively boost involvement from underrepresented populations in clinical trials, it is essential to utilize targeted recruitment strategies. Establishing collaborations with community organizations, such as Acclinate—a company dedicated to enhancing diversity in research—provides valuable insights and access to underrepresented populations in clinical trials. Additionally, employing culturally relevant messaging ensures that communications resonate with potential participants, fostering trust and engagement. Offering flexible participation options, including remote consultations, accommodates varying needs and preferences.
Compensation for time and travel is another critical factor that alleviates financial barriers, making participation more feasible for individuals from diverse backgrounds. A recent initiative demonstrated a 42% increase in screenings for an infant formula study within just 60 days by employing targeted outreach strategies.
By actively engaging with communities and understanding their distinct needs, researchers can cultivate a more inclusive atmosphere that promotes diverse involvement in health studies. Statistics indicate that over 80% of patients are unaware of research studies or how to participate, highlighting the necessity for proactive outreach. Establishing trust through ongoing community involvement is essential, as it not only enhances recruitment rates but also improves the overall quality and reliability of research outcomes.
To achieve significant advancement in enhancing diversity within medical trials, organizations must implement systemic changes that reflect a genuine commitment to inclusivity for underrepresented populations in clinical trials. This involves:
Policies should prioritize inclusive practices, ensuring that diverse perspectives are not only welcomed but actively sought. Leadership plays a crucial role in this transformation; by fostering a culture of diversity and inclusion, they can ensure these values permeate every aspect of research in the healthcare field. Such an environment not only enhances the research process but also significantly elevates the quality and relevance of results, ultimately benefiting underrepresented populations in clinical trials.
The FDA plays a pivotal role in enhancing diversity in medical studies through its regulatory guidance and initiatives. Recent mandates require research study sponsors to submit Diversity Action Plans that outline strategies for including underrepresented populations in clinical trials. By establishing explicit standards for diversity in study design and participant selection, the FDA encourages researchers to prioritize inclusivity. Compliance with these regulations not only elevates the ethical standards of clinical research but also enhances the generalizability of trial results, ultimately benefiting public health.
Moreover, sponsors must communicate if their inclusion objectives are deemed unfeasible and provide justifications for this assessment, ensuring accountability within the regulatory framework. The requirement for sponsors to present an action plan related to underrepresented populations in clinical trials at the time of submitting their protocol for phase 3 or pivotal studies is a significant aspect of the FDA's expectations.
Successful Diversity Action Plans have illustrated the effectiveness of engaging diverse investigators and community organizations, significantly bolstering recruitment efforts for underrepresented populations in clinical trials. For instance, researchers who cultivate relationships with local community leaders, such as religious figures, have reported increased participation from historically excluded populations in underrepresented populations clinical trials. This strategy not only enhances enrollment but also fosters trust between researchers and communities.
Additionally, sponsors must elucidate how they plan to achieve inclusion objectives, detailing outreach strategies and study-site selection, as specified in the FDA's guidance. Here, bioaccess can play a crucial role by offering comprehensive research study management services, including feasibility assessments, site selection, compliance evaluations, and project oversight, ensuring that studies are effectively designed to meet inclusivity goals.
The FDA also underscores the importance of broadening eligibility standards and preventing unwarranted exclusions to enhance diversity in underrepresented populations in clinical trials. As the FDA continues to refine its regulations, the emphasis on diversity in research studies is expected to grow, with further guidance anticipated on the content and structure of Diversity Action Plans. This ongoing development underscores the FDA's commitment to ensuring that research studies accurately represent the diverse groups they aim to assist, ultimately leading to more effective and inclusive healthcare solutions.
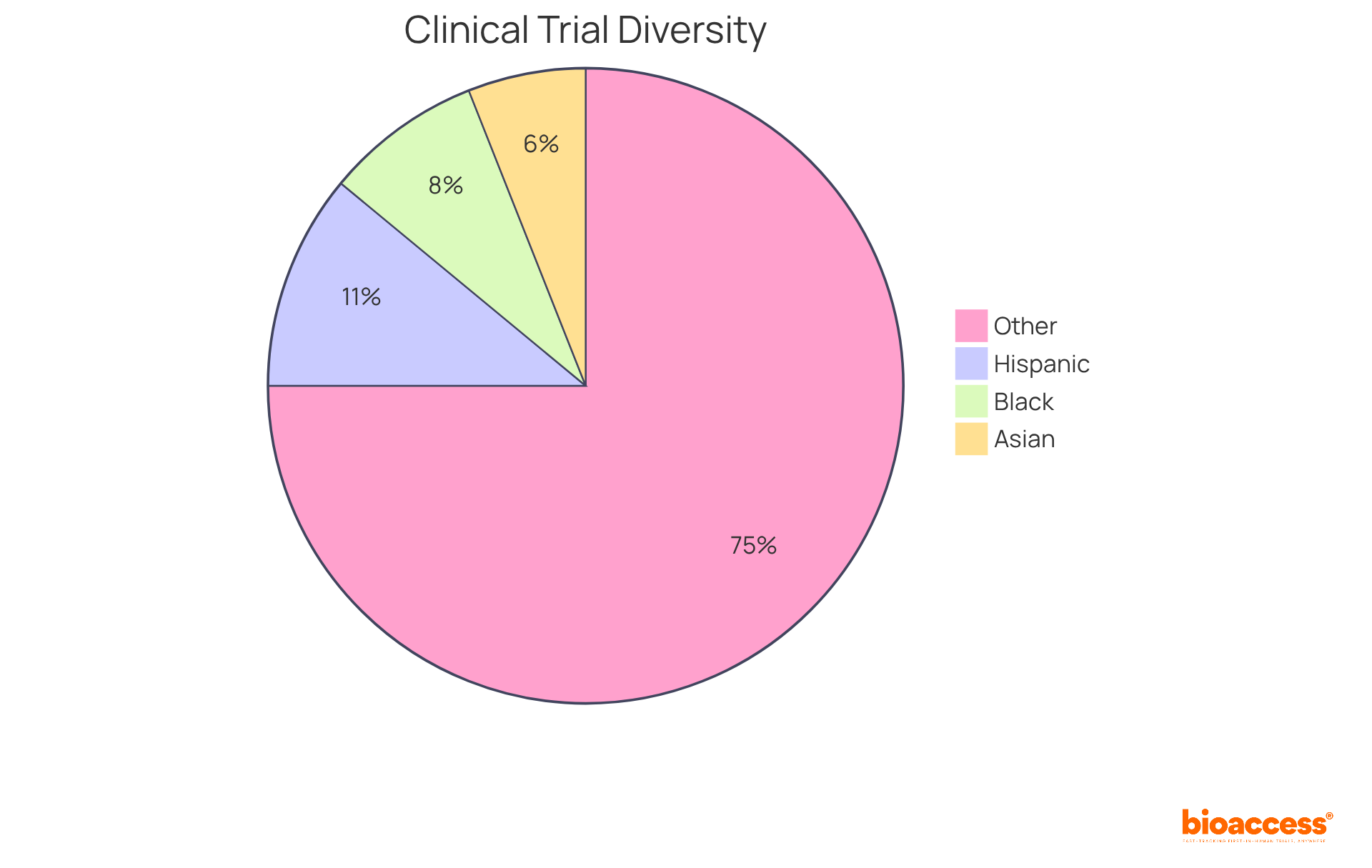
Inclusivity in clinical studies is crucial for ensuring that new medications are effective across various groups. Genetic, lifestyle, and environmental variations significantly influence individual responses to medications. For instance, in 2020, only 8% of individuals in new drug trials in the US were Black, 6% were Asian, and 11% were Hispanic, despite racial and ethnic minorities comprising 39% of the U.S. demographic. This underrepresentation raises concerns about the safety and efficacy of treatments for these groups.
By incorporating a broad spectrum of participants, researchers can collect data that accurately reflects these differences, leading to more effective and safer treatments for all demographic groups. This method not only enhances the scientific credibility of studies but also promotes health equity, ensuring that all groups benefit from medical progress.
The FDA mandates the inclusion of diverse demographics in drug testing, as neglecting this can result in ineffective or harmful medications for underrepresented populations. Historical mistrust, such as that stemming from the Tuskegee Syphilis Experiment, which resulted in the deaths of over 200 participants, highlights the need for ethical practices and community engagement to rebuild trust and encourage participation.
As Stephanie Monroe, Executive Director of AfricanAmericansAgainst Alzheimer’s, stated, "When you don’t have inclusion of diverse communities, you run the risk of making assumptions about drug safety and effectiveness that may not be accurate."
Ultimately, diversity in medical studies is essential for enhancing drug safety and effectiveness, establishing it as a critical focus for the future of medical research. Clinical research professionals should actively seek partnerships with community organizations to improve recruitment efforts and foster trust.
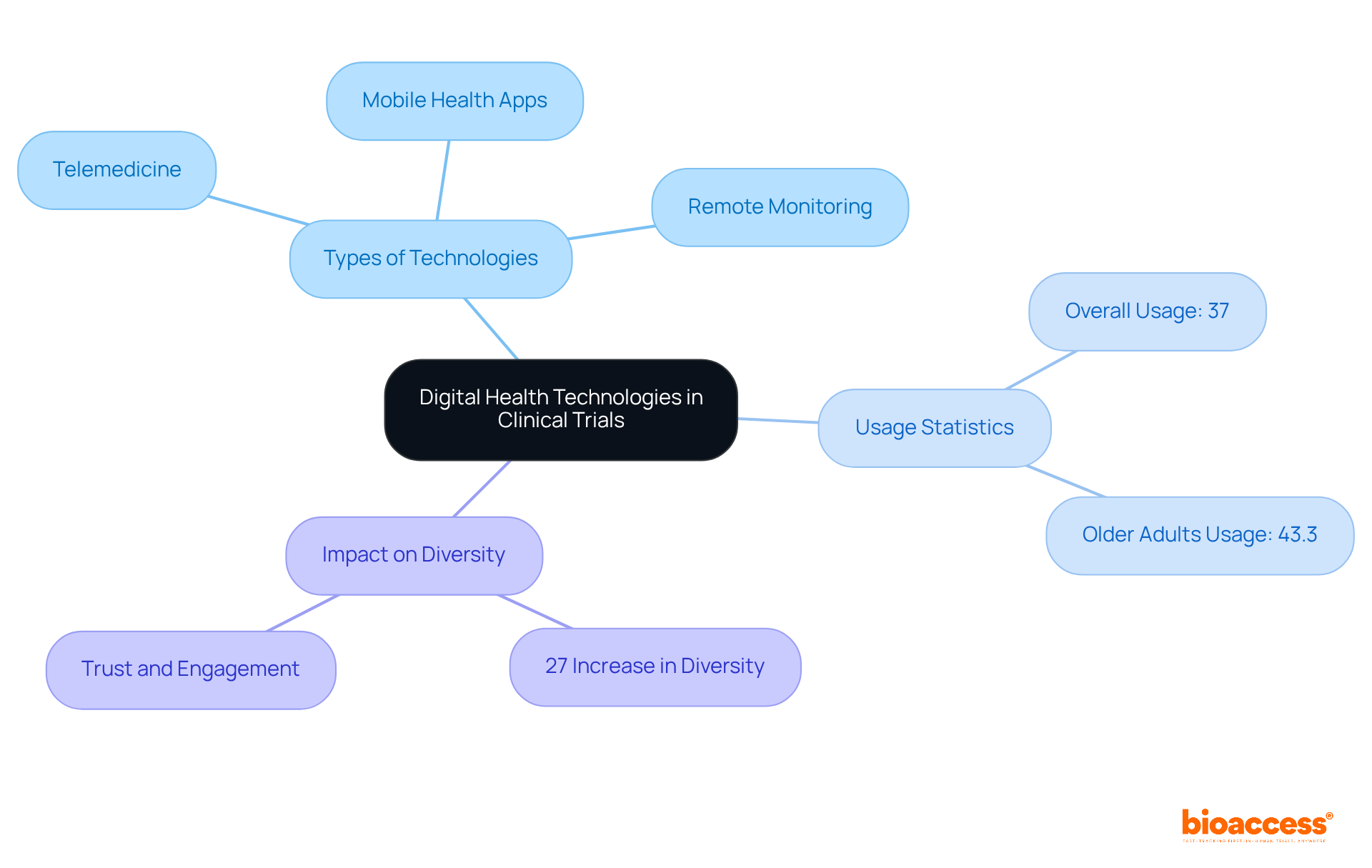
Digital health technologies are fundamentally transforming the research landscape, enhancing access and engagement for diverse populations. Telemedicine, mobile health applications, and remote monitoring devices are pivotal in engaging participants who encounter barriers to traditional study involvement. Notably, the use of telemedicine has surged, with 37% of adults over 18 utilizing these services in the past year; this figure escalates among older adults, reaching 43.3% for those aged 65 and above.
By harnessing these technologies, medical studies can achieve greater inclusivity in underrepresented populations clinical trials, resulting in a 27% increase in participant diversity. Furthermore, digital platforms enable real-time data collection and enhance communication with participants, which is vital for fostering trust and engagement. This evolution not only enhances the representation of marginalized groups in underrepresented populations clinical trials but also leads to more efficient and effective research outcomes, ultimately advancing health equity in medical research.
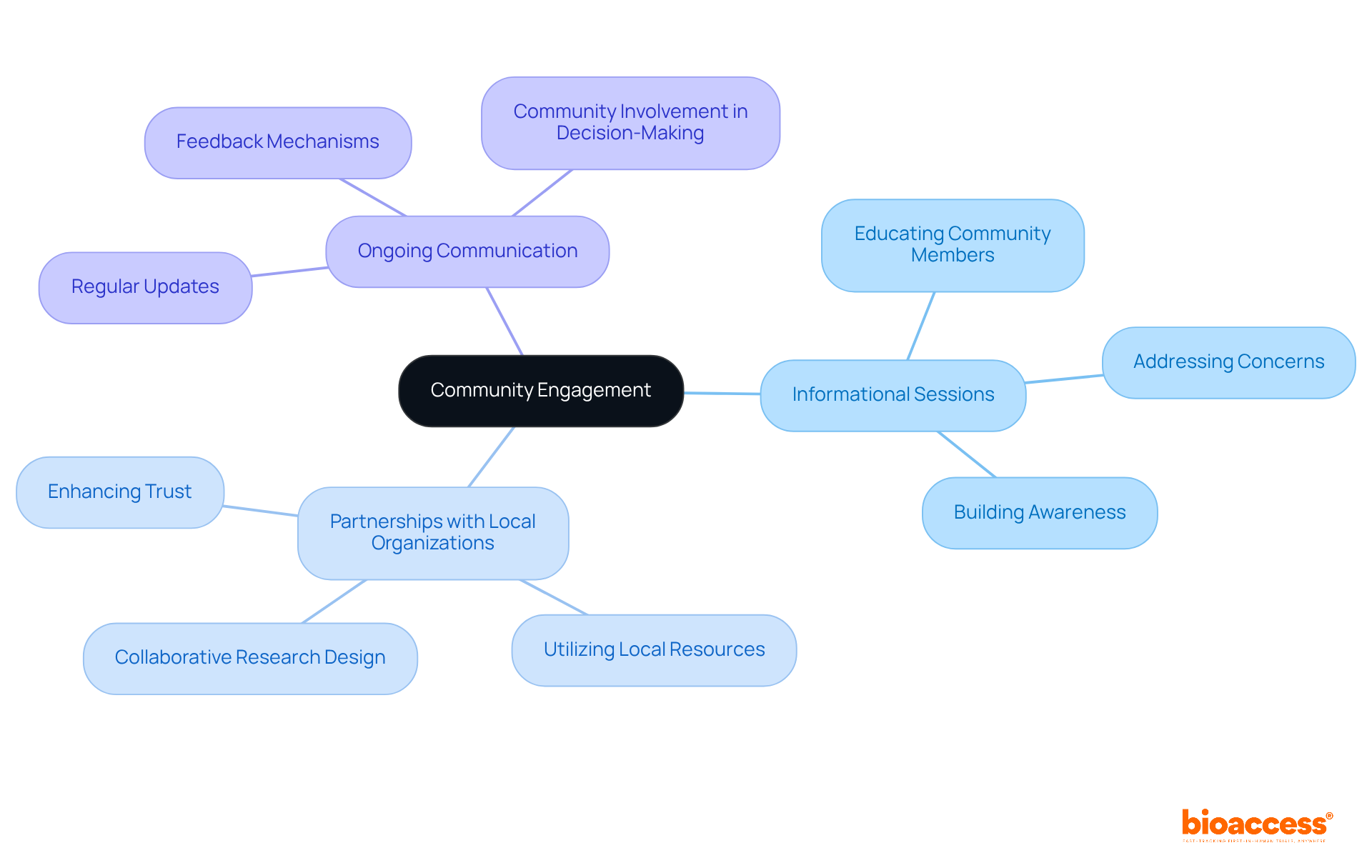
Establishing confidence through community involvement is essential for boosting participation in research studies. Researchers must actively engage community members in both the planning and execution of studies, ensuring their voices are heard and concerns are addressed. This engagement can be achieved through:
Collaborative partnerships have proven effective; they ensure that studies are tailored to community needs, which leads to increased trust and participation. Ultimately, these efforts culminate in more varied and representative medical studies, with a focus on underrepresented populations in clinical trials, addressing historical inequalities in research involvement.
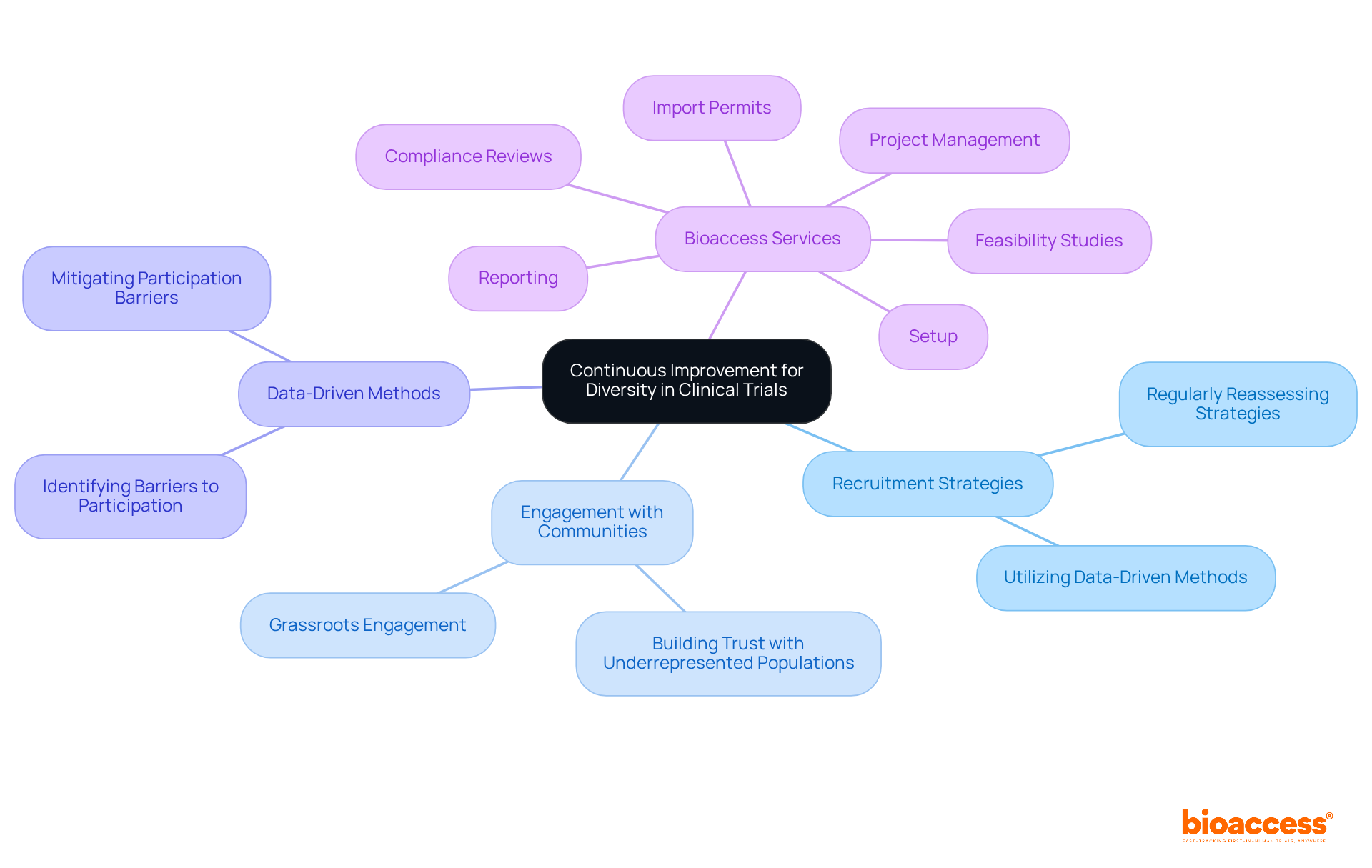
The future of diversity in medical studies hinges on a steadfast commitment to continuous improvement and adaptation. As demographics evolve and new technologies arise, it is imperative for researchers to proactively enhance inclusivity. This involves:
Bioaccess is dedicated to this mission, offering comprehensive research management services that encompass:
By fostering a culture of innovation and inclusivity, the research community can guarantee that studies are representative and that their findings benefit all populations. Moreover, the impact of Medtech clinical studies transcends the trials themselves, bolstering local economies through job creation, economic growth, and healthcare enhancements, while fostering international collaboration. With bioaccess at the forefront in Latin America, the emphasis on regulatory excellence and innovation is essential for advancing global health improvement.
Enhancing diversity in clinical trials stands as both a moral imperative and a scientific necessity. The inclusion of underrepresented populations ensures that medical research accurately reflects the realities of diverse demographics, ultimately leading to safer and more effective treatments. By addressing historical mistrust, implementing targeted recruitment strategies, and utilizing digital health technologies, the research community can cultivate a more inclusive environment that benefits all.
This article has discussed key strategies, emphasizing the importance of community engagement, the role of regulatory guidance from the FDA, and the need for systemic changes within organizations. These approaches not only enhance participation from diverse groups but also improve the validity of research outcomes. The insights provided illustrate how a concerted effort can dismantle barriers and build trust, paving the way for equitable healthcare advancements.
The future of clinical trials hinges on a commitment to continuous improvement and adaptation. As the healthcare landscape evolves, so too must the strategies employed to ensure representation of all populations in research. By prioritizing diversity and inclusivity, the medical community can enhance treatment efficacy and promote health equity, ultimately leading to a healthier society for everyone.
What is bioaccess® and how does it enhance diversity in clinical trials?
bioaccess® is an organization that leverages its operational advantages in Latin America, particularly Colombia, to enhance diversity in clinical trials by recruiting underrepresented populations. It capitalizes on Colombia's cost savings, efficient regulatory review process, and a well-regarded healthcare system to accelerate recruitment.
What competitive benefits does Colombia offer for clinical trials?
Colombia offers cost savings exceeding 30% compared to North America and Western Europe, a regulatory review process that typically takes 90 to 120 days, and a healthcare system ranked 22nd by the World Health Organization. Additionally, about 95% of Colombia's population is covered by universal healthcare, providing a diverse patient base for recruitment.
Why is racial diversity important in clinical trials?
Racial diversity is crucial because genetic, environmental, and lifestyle factors can significantly affect individual responses to medications. Including diverse participants helps researchers understand these variations, leading to more effective and tailored healthcare solutions.
How does diversity impact trial outcomes?
Trials that include diverse populations yield results that are more generalizable and applicable to the broader population. Research shows that representation can increase interest and trust among participants, which enhances recruitment and the overall validity of study findings.
What historical factors contribute to the mistrust of minority groups in clinical trials?
Historical occurrences, such as the Tuskegee Syphilis Study, have created deep-seated distrust among minority communities towards medical studies. Ongoing disparities in healthcare access and treatment outcomes further exacerbate this mistrust.
What strategies can researchers use to improve participation from underrepresented populations?
Researchers can improve participation by recognizing historical contexts, fostering trust through transparent communication, and employing community-based participatory research (CBPR) models that prioritize cultural awareness and respect.
What are the potential economic impacts of health inequities in clinical trials?
The lack of representation in medical studies could lead to significant economic burdens, with projections indicating that health inequities could cost society over $5 trillion for diabetes alone by 2050.