


The article focuses on the various clinical data management systems that enhance research efficiency, highlighting nine systems that transform clinical research practices. It emphasizes that these systems, such as bioaccess®, Medidata Rave EDC, and LabKey, improve patient enrollment, data quality, and compliance. This ultimately leads to faster and more reliable research outcomes, supported by evidence of reduced entry mistakes and improved decision-making capabilities. The significance of these advancements cannot be overstated, as they address critical challenges within the Medtech landscape. Collaboration among stakeholders will be essential in maximizing the benefits of these systems, paving the way for innovative solutions in clinical research.
Clinical data management systems are revolutionizing the landscape of medical research, effectively addressing critical inefficiencies that have long plagued the industry. These advanced tools not only streamline data collection and analysis but also enhance compliance and data integrity, ultimately leading to faster and more reliable research outcomes. As the demand for innovative solutions grows, researchers now face the challenge of navigating a rapidly evolving technological landscape.
What key systems are transforming research efficiency today, and how can organizations leverage these advancements to maintain a competitive edge in the field of clinical trials?
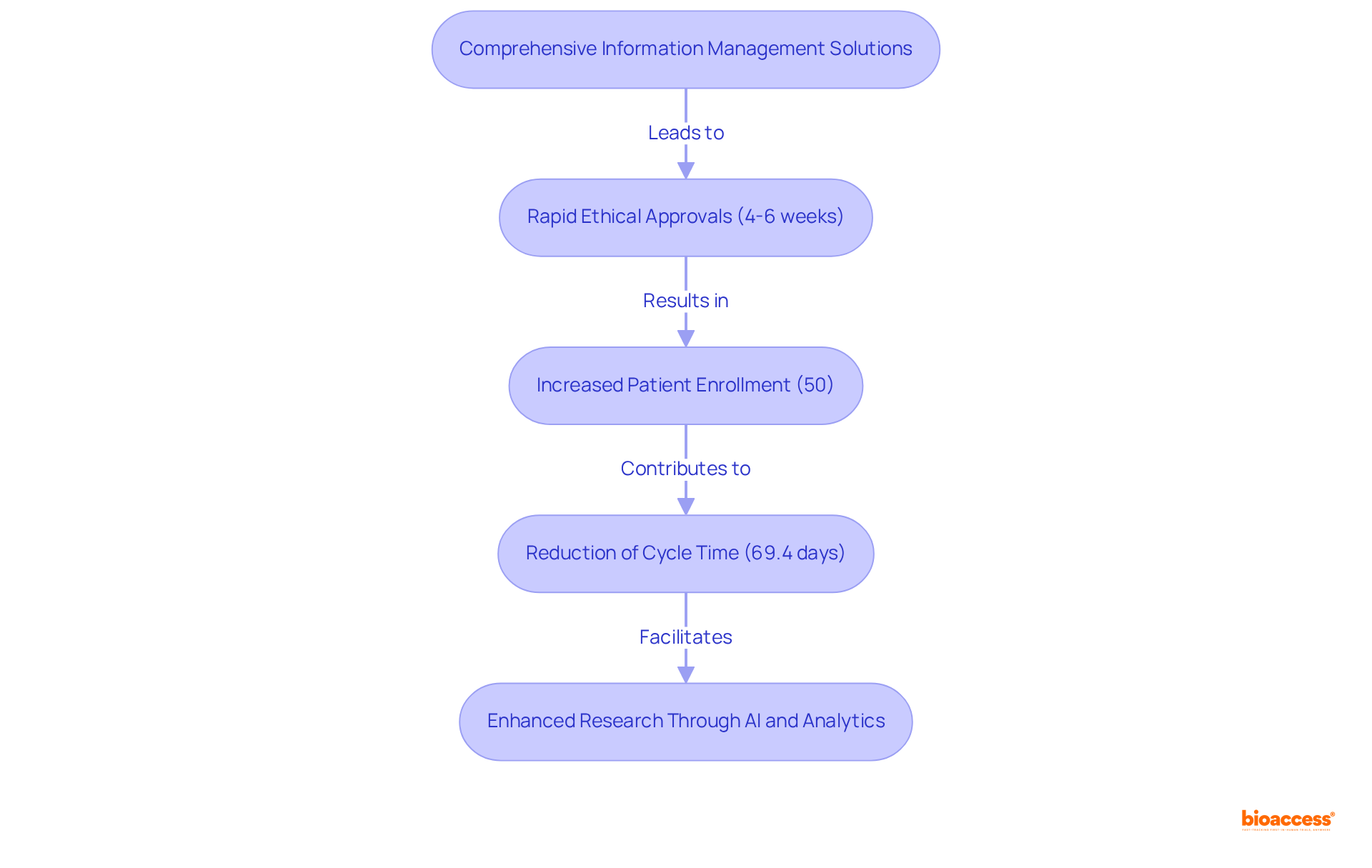
bioaccess® excels in delivering comprehensive information management solutions that significantly enhance the efficiency of research processes. By leveraging Latin America's regulatory speed and diverse patient populations, bioaccess® secures ethical approvals in an impressive 4-6 weeks. This rapid turnaround not only increases patient enrollment by 50% compared to traditional markets but also upholds rigorous standards of integrity and compliance.
As a result, bioaccess® has become a preferred partner for Medtech, Biopharma, and Radiopharma innovators seeking to streamline their research studies. Industry leaders emphasize that effective information management is crucial for reducing the average cycle time from final protocol approval to database launch, currently at 69.4 days—indicating a critical area for improvement.
Through the implementation of advanced data management systems, bioaccess® directly addresses these challenges, ensuring that medical studies are not only expedited but also more reliable. The integration of AI and analytics into their operations further positions bioaccess® at the forefront of research innovation, allowing for a more agile response to the evolving demands of the healthcare landscape.
As Allison Dunn, Founder of Deliberate Directions, notes, 'AI is enhancing diagnostics, automating administrative tasks, and improving personalized treatment plans,' underscoring the transformative potential of technology in healthcare research.
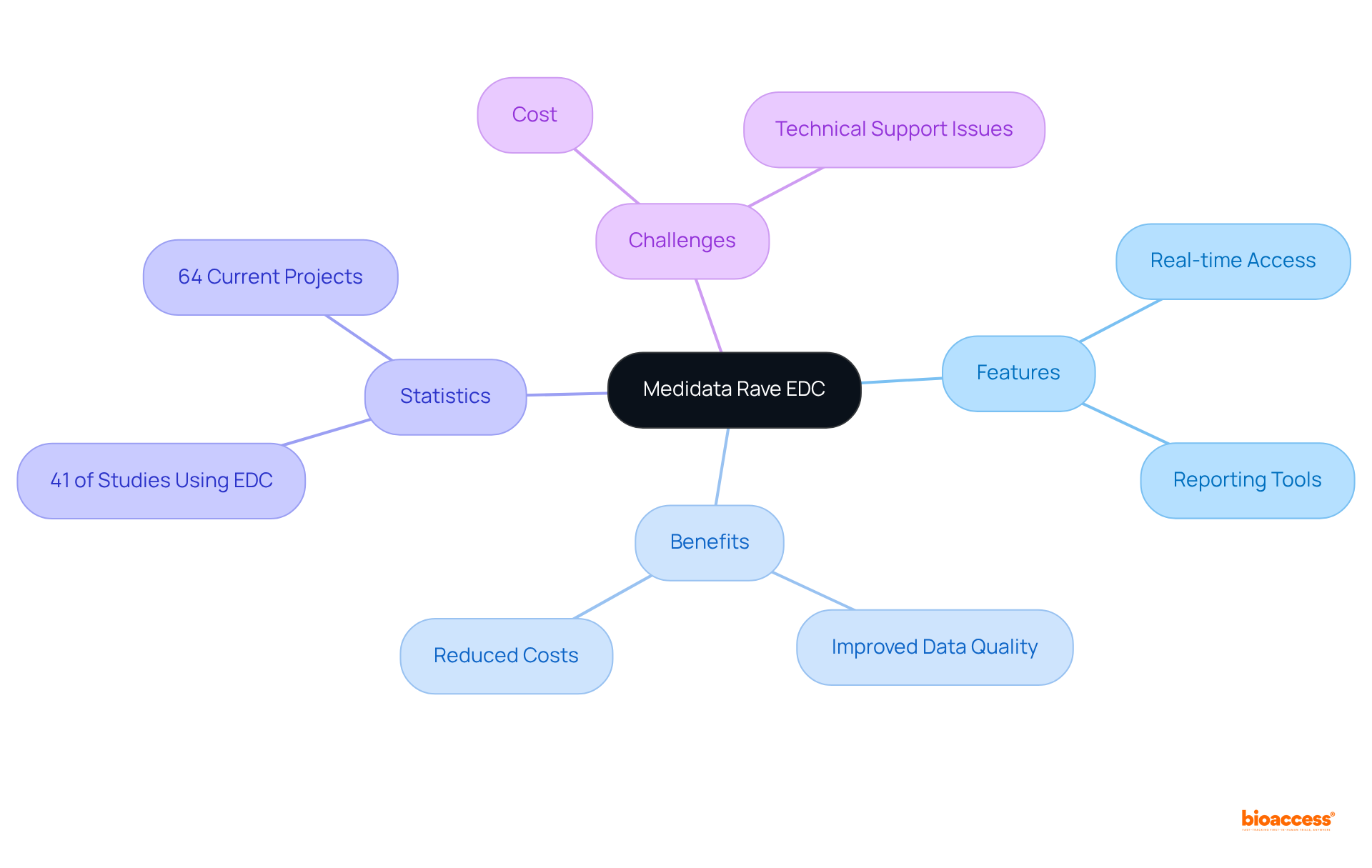
Medidata Rave EDC stands out as a premier electronic information capture system, meticulously crafted to boost the efficiency of research trials. Its intuitive interface and robust information management capabilities empower researchers to capture, manage, and analyze research data with ease. Notable features include:
Research indicates that employing clinical data management systems, such as Medidata Rave, can lead to improved data quality and reduced costs, solidifying its status as an indispensable asset in the research landscape. As of 2007, approximately 41% of studies were leveraging an Electronic Data Capture (EDC) system, reflecting the increasing recognition of these tools. Furthermore, bioaccess® ensures that enrollment occurs 50% faster than traditional markets, highlighting the efficiency that EDC systems can bring to research endeavors. Currently, the EDC system is actively supporting 64 projects, demonstrating its practical application in ongoing research.
Nevertheless, challenges to the adoption of clinical data management systems, including cost and insufficient technical support, persist as critical factors for researchers to consider.
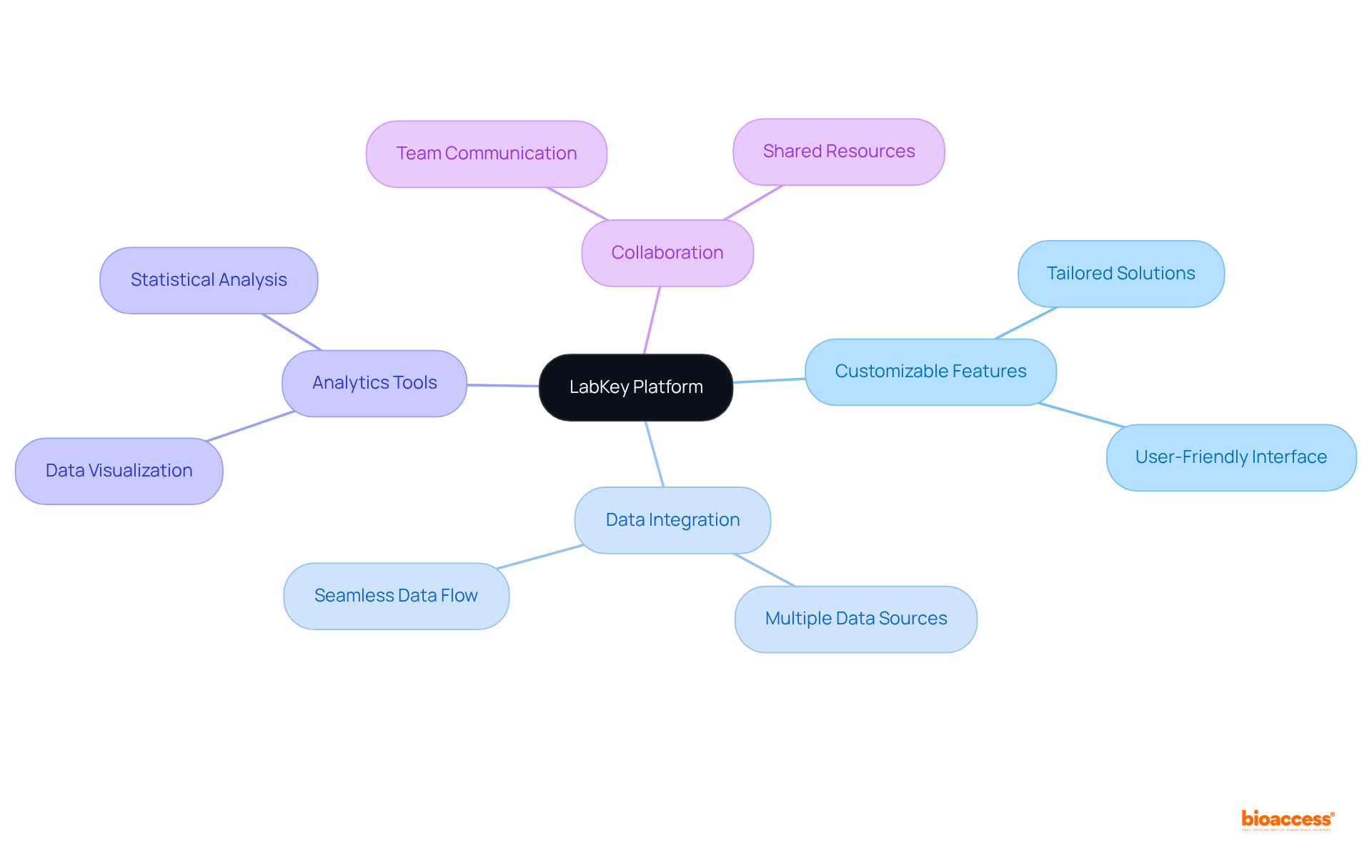
LabKey offers a versatile platform for managing research information, including clinical data management systems, to meet the intricate requirements of scientists in the clinical research landscape. Its customizable features facilitate the incorporation of various information types and sources, making it particularly suited for studies that demand complex information handling. Furthermore, LabKey's powerful analytics tools empower researchers to derive significant insights from their data, thereby promoting improved decision-making and enhancing the overall quality of medical research. This integration of capabilities underscores the importance of collaboration in navigating the challenges faced in the Medtech sector.
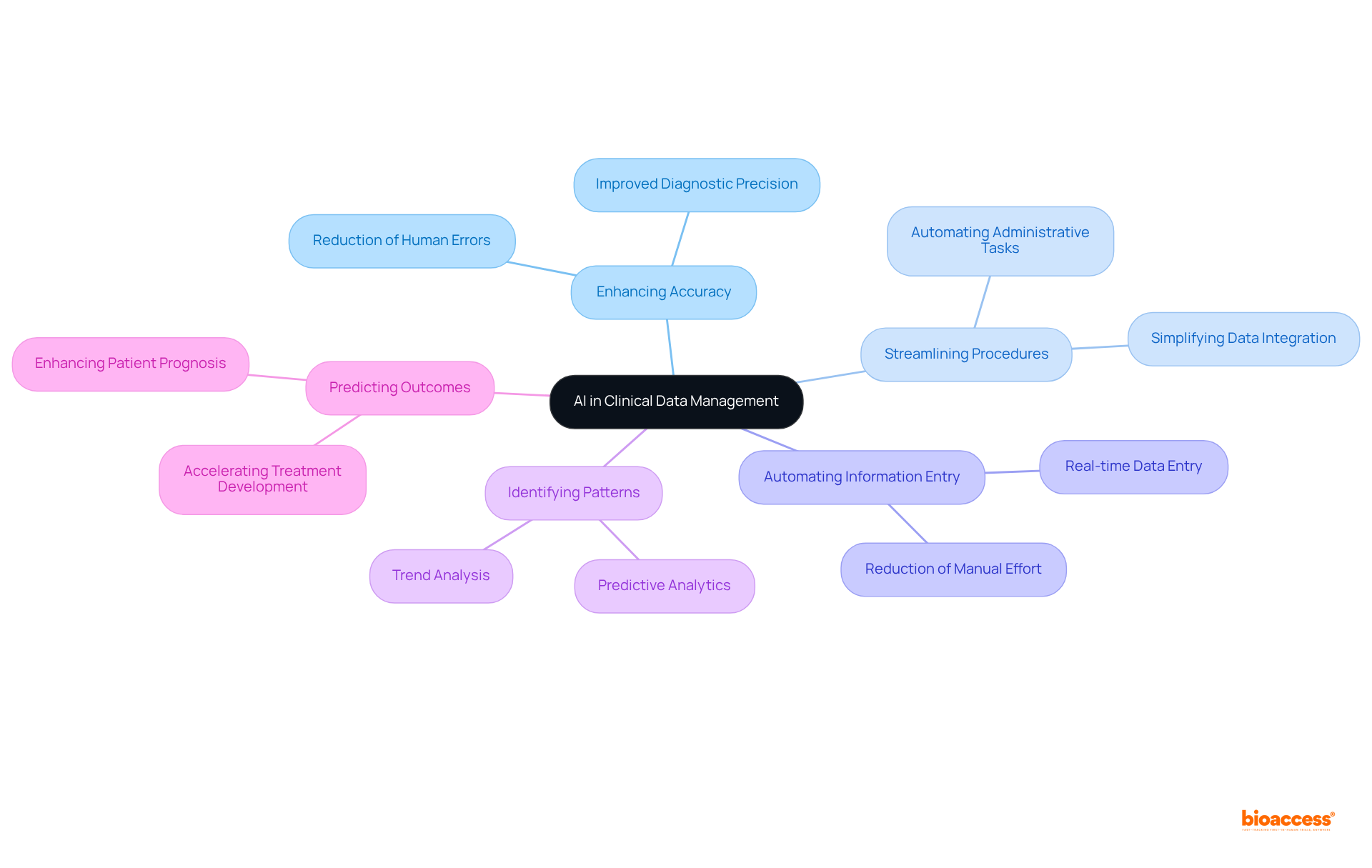
Dove Press explores the transformative impact of artificial intelligence (AI) in healthcare information oversight, highlighting its potential to enhance accuracy and streamline procedures. AI technologies are capable of automating information entry, identifying patterns, and predicting outcomes in clinical data management systems, which significantly reduces the time and effort required for data management. By integrating AI into medical studies, researchers can improve efficiency, minimize human errors, and ultimately accelerate the development of new treatments.
GMI Insights provides a comprehensive examination of the healthcare information systems market, highlighting critical trends, challenges, and opportunities that shape the industry. This analysis is essential for stakeholders seeking effective solutions tailored to their specific needs, empowering them to maintain a competitive edge in a rapidly evolving landscape.
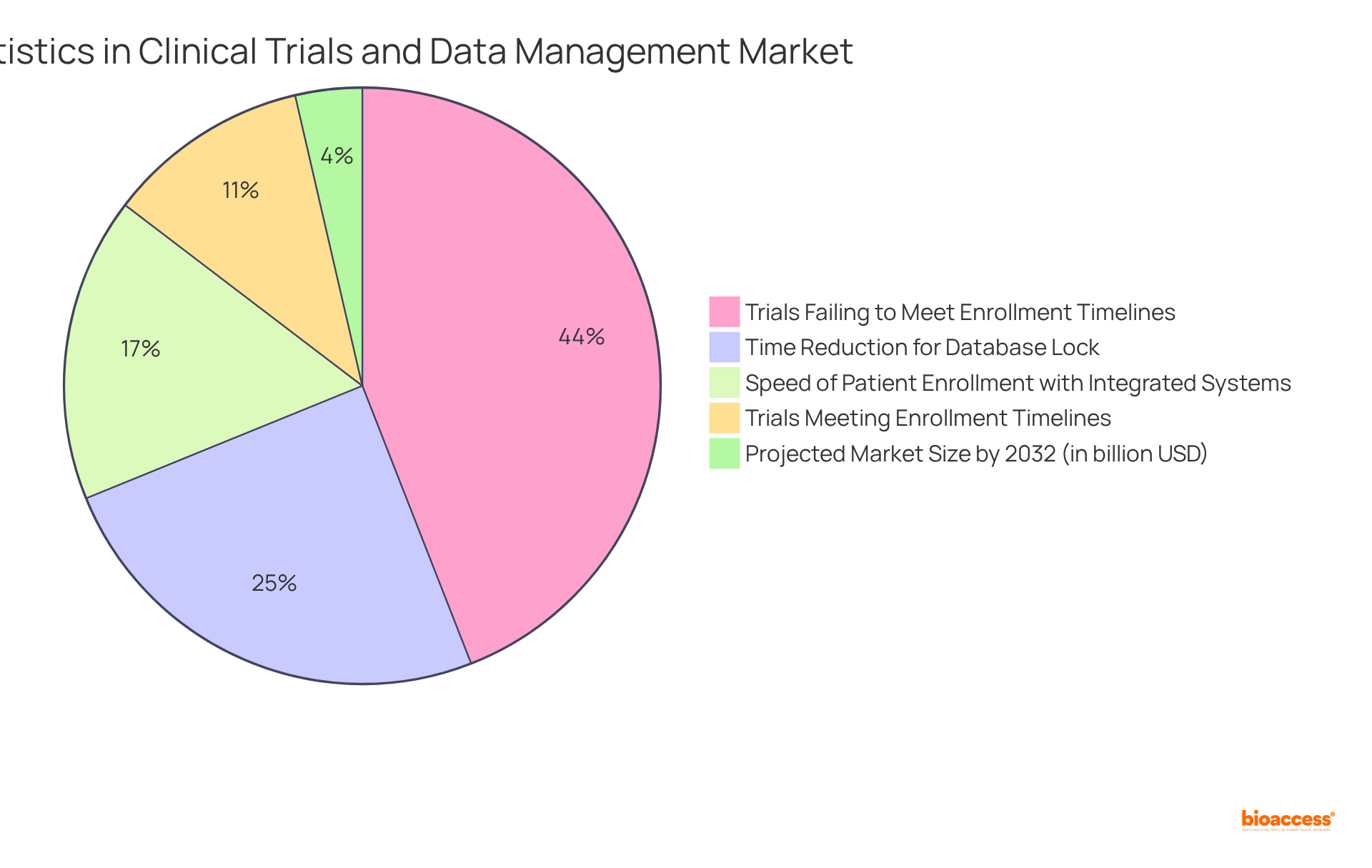
For instance, nearly 80% of clinical trials fail to meet enrollment timelines, underscoring the urgent need for robust information handling strategies. Organizations can leverage integrated, automated clinical data management systems to address this issue, as these systems not only expedite patient enrollment by 30% but also achieve database lock in 45% less time compared to fragmented systems. Furthermore, pharmaceutical companies that implement comprehensive clinical data management systems increase their likelihood of regulatory approval by 23%.
By understanding these market dynamics, organizations can strategically position themselves to adopt the right clinical data management systems and methodologies, ultimately enhancing the efficiency and success of their trials. The Clinical Information Handling Systems market is projected to grow significantly, reaching approximately 6.57 billion USD by 2032, reflecting the increasing demand for efficient information handling solutions.
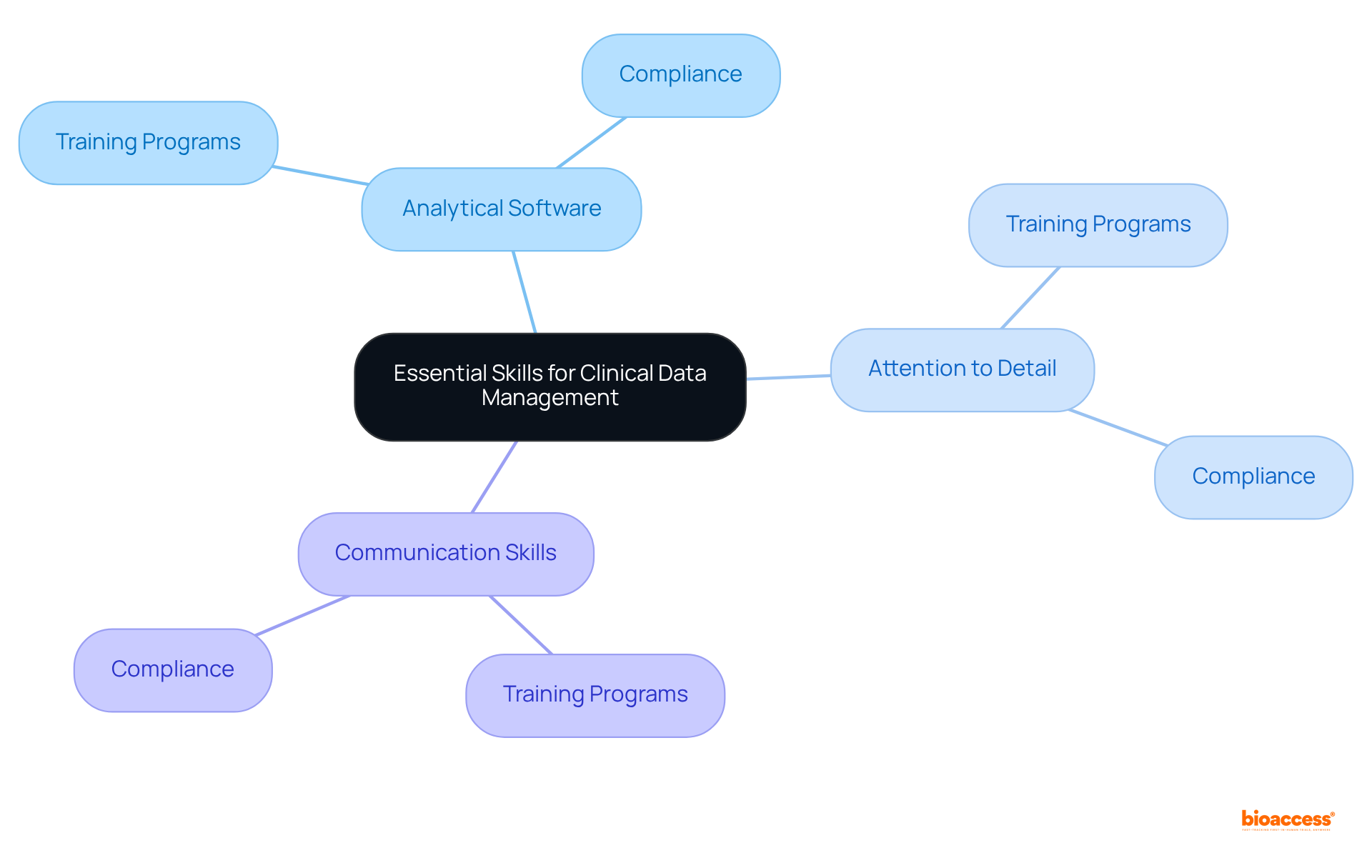
Efficient handling of healthcare information is paramount, relying on a blend of vital abilities and instruments. Mastery of analytical software, meticulous attention to detail, and strong communication skills are essential for maintaining information integrity and ensuring compliance throughout the clinical study process. Organizations that invest in equipping their teams with the right tools and comprehensive training programs can significantly enhance their information management practices. Notably, training programs focused on information management and regulatory adherence have been proven to improve information handling and reporting accuracy, ultimately leading to better study outcomes.
As the landscape of medical research evolves, prioritizing these competencies becomes crucial for organizations aiming to successfully navigate the complexities of contemporary trials. With job opportunities in areas related to medical research and information oversight projected to increase by 17% by 2031, the importance of proficient healthcare information handling skills is undeniable. Furthermore, as organizations generate information at an unprecedented pace—comparable to the total volume of the Library of Congress's entire print collection three times each second—robust training programs in information handling are essential.
Bioaccess®, boasting over 15 years of expertise in research, underscores the necessity of these competencies to ensure successful trial results.
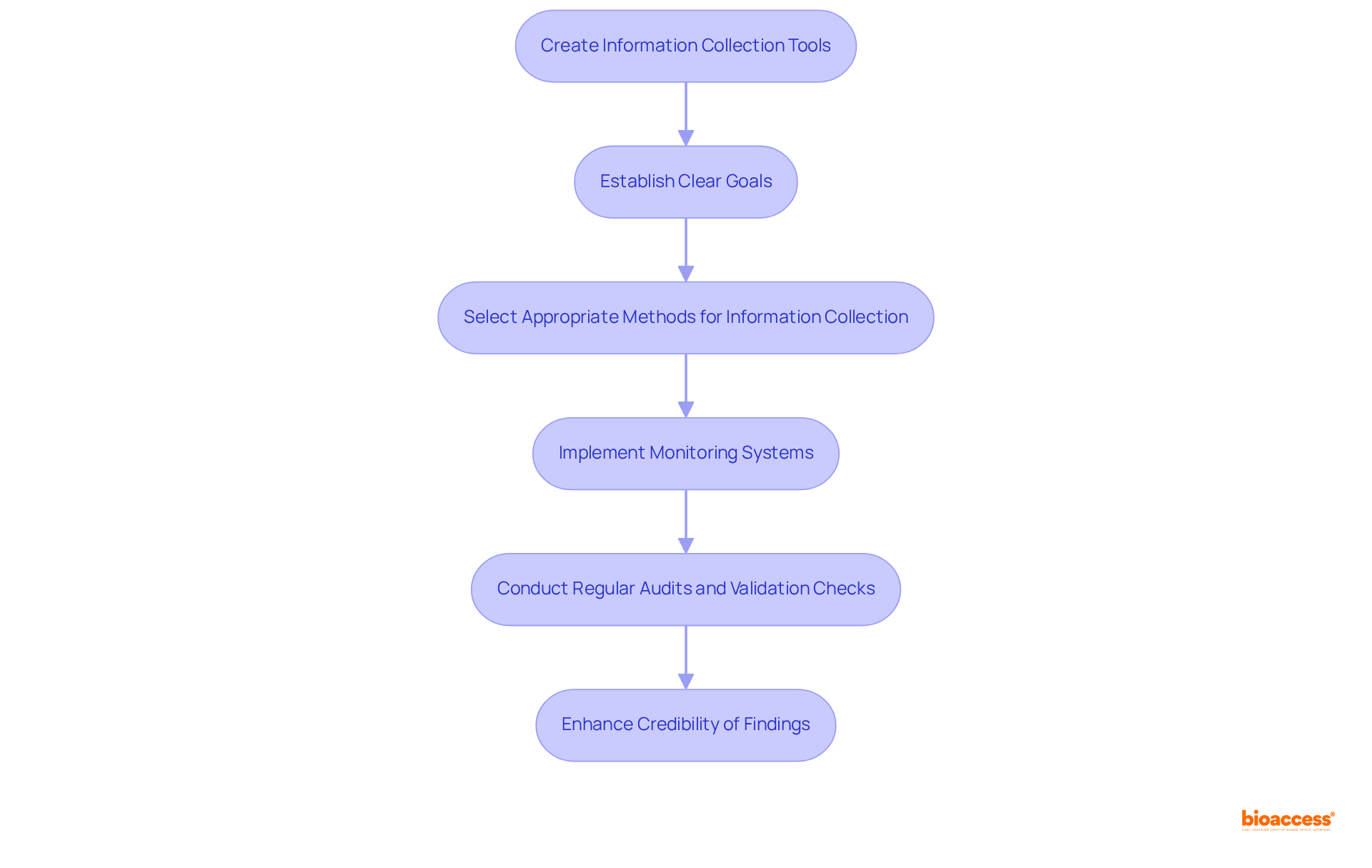
Effective clinical data management systems are essential for ensuring the reliability and validity of research findings. The process begins with the creation of information collection tools tailored to the specific requirements of the study. This includes establishing clear goals and selecting appropriate methods for precise information collection. Ensuring information quality is paramount; researchers must implement robust monitoring systems to track integrity throughout the study. This necessitates regular audits and validation checks to minimize errors and uphold high standards.
Statistics reveal that medical studies utilizing integrated information systems can achieve a 40% reduction in entry mistakes and a three-month acceleration in database closure time, as evidenced by a Phase III oncology study. Furthermore, organizations prioritizing information quality assurance report significant enhancements in overall experiment efficiency, with a 28% improvement in quality metrics observed in studies employing AI-driven solutions. Additionally, pharmaceutical companies that adopt strong clinical data management systems reduce clinical trial costs by an average of 25%.
Citations from industry experts highlight the importance of rigorous information gathering practices. For instance, Joo Ann Lee emphasizes that "data science isn't about the amount of information but rather the quality," underscoring the necessity of focusing on key elements that yield valuable insights. Moreover, Matthew Emerick notes, 'Information is the nutrition of artificial intelligence,' stressing that high-quality information is crucial for effective analysis and decision-making.
By adhering to established information handling procedures, researchers can not only enhance the credibility of their findings but also make significant contributions to the advancement of medical knowledge and the successful commercialization of innovative products.
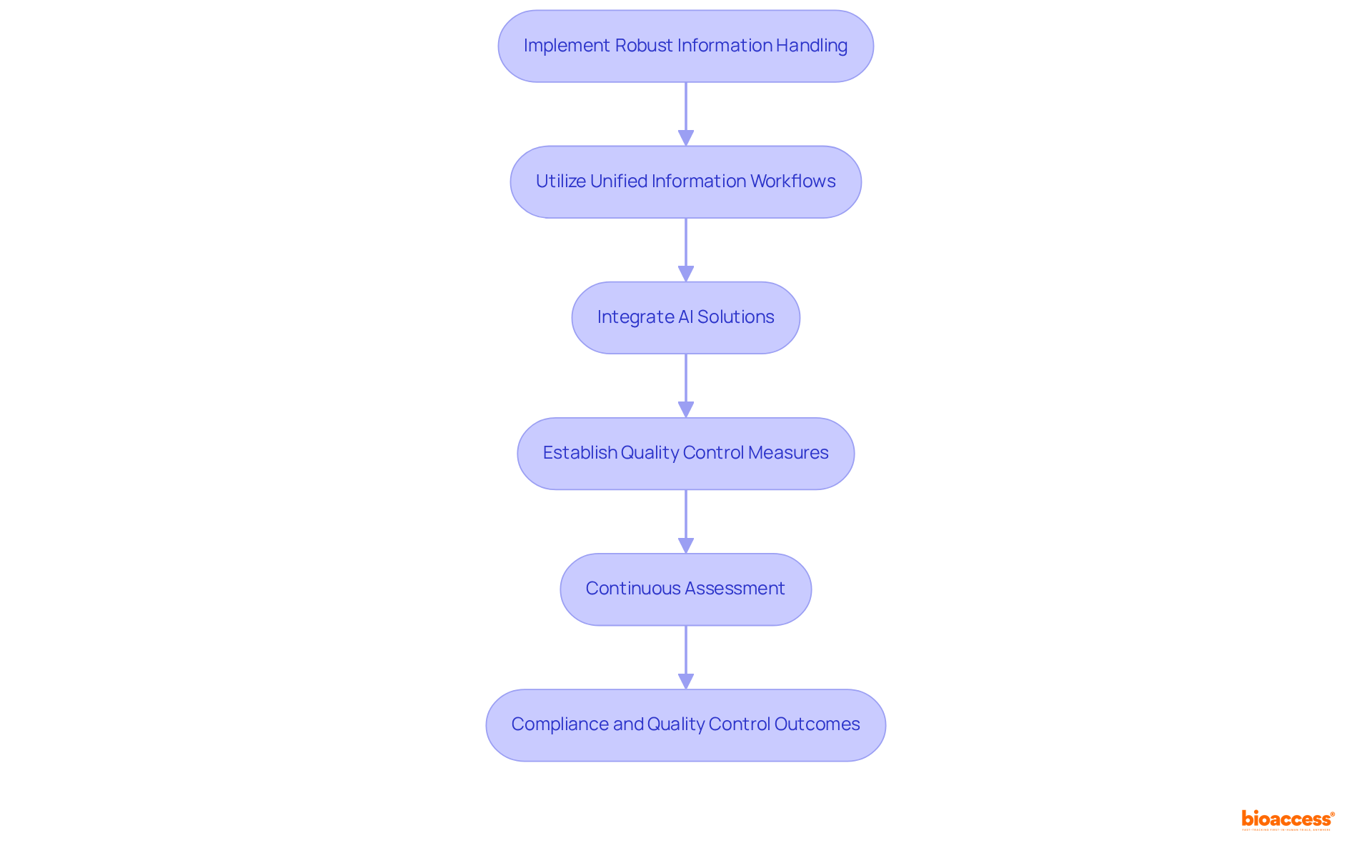
Clinical data management systems are essential for ensuring adherence to regulatory standards and maintaining quality control throughout the research process. By implementing robust information handling practices and utilizing clinical data management systems, organizations can significantly reduce errors and enhance information integrity, ensuring compliance with Good Clinical Practice (GCP) guidelines for all studies. This commitment to compliance not only protects patient safety but also fosters trust among stakeholders.
For instance, experiments utilizing unified information processing workflows complete patient enrollment 30% faster and achieve database lock in 45% less time than those relying on fragmented systems. Moreover, clinical trials that employ clinical data management systems integrated with AI-driven information handling solutions have reported a 35% reduction in query resolution time, underscoring the effectiveness of modern information systems.
Additionally, establishing quality control measures, such as regular information audits and automated validation checks, can lead to a 28% improvement in quality metrics. Industry leaders stress the importance of a proactive approach to quality control; as Paul Koziarz asserts, "Businesses have to be in a constant state of remediation and education." Organizations must continuously assess and refine their processes to keep pace with evolving regulatory requirements and technological advancements.
Furthermore, addressing challenges in compliance oversight, such as training employees on policies and aligning practices with changing regulations, is crucial for sustaining effective quality control. By prioritizing quality assurance in research oversight, organizations can enhance trial outcomes and accelerate the path to regulatory approval.
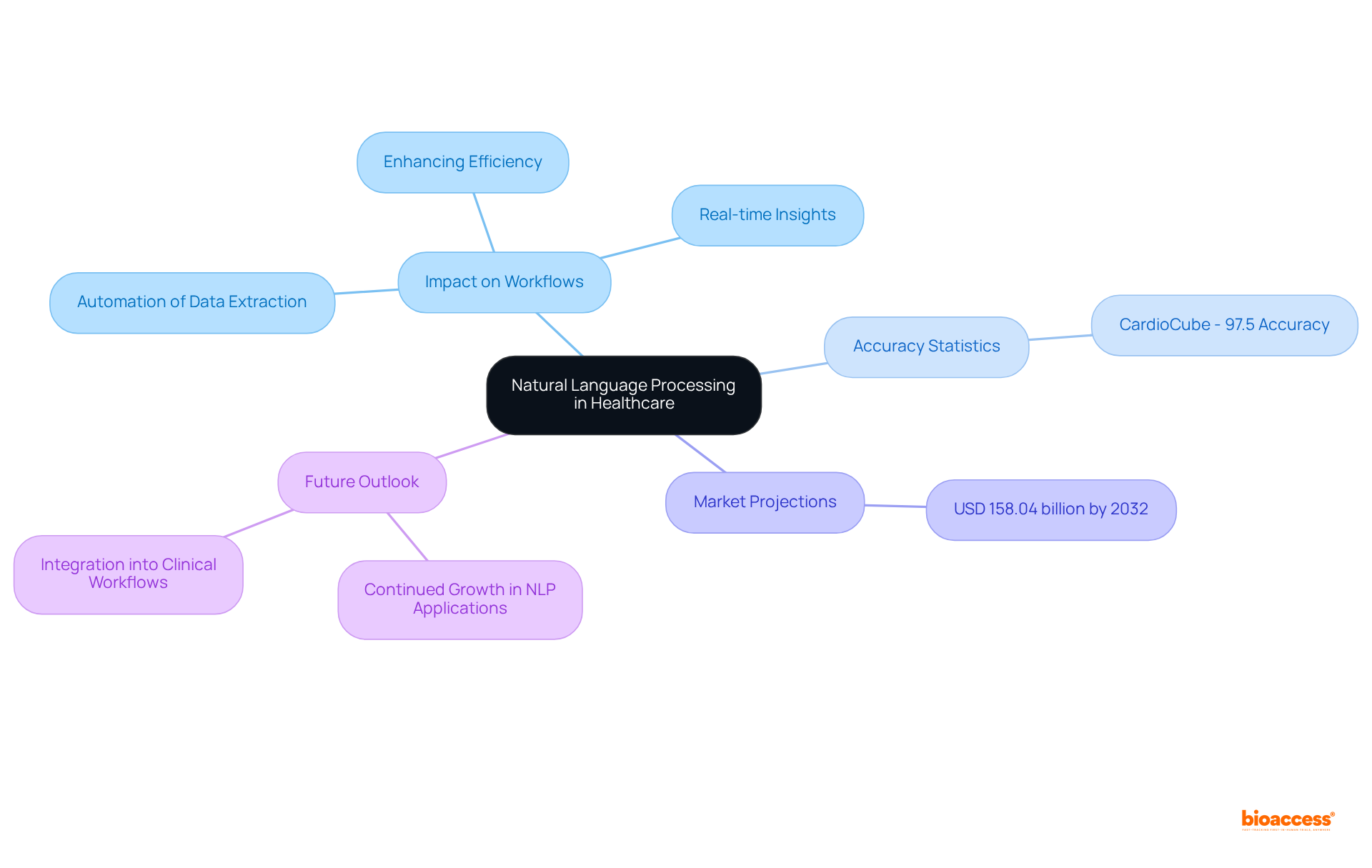
Natural Language Processing (NLP) is fundamentally transforming healthcare information management workflows by automating the extraction and analysis of unstructured information. This technology is reshaping the landscape of clinical research by converting free-text medical notes into structured formats, significantly enhancing data processing efficiency and accuracy in information retrieval.
For instance, the CardioCube software achieved an impressive 97.5% accuracy rate in collecting cardiovascular risk factors and medical history, highlighting the potential of NLP in healthcare environments. Such advancements empower researchers to concentrate on advanced analysis and strategic decision-making, ultimately leading to more effective medical studies.
Looking ahead, by 2025, progress in NLP technology is expected to further refine these processes, with the global NLP market projected to reach USD 158.04 billion by 2032, underscoring its growing significance in medical research. Investigators have noted that NLP not only accelerates information processing but also facilitates real-time insights, enabling quicker responses to emerging trends and challenges within studies. This capability is crucial in today’s fast-paced research environment, where timely and accurate information can significantly influence study outcomes.
To harness these advancements, medical researchers should consider integrating NLP tools into their workflows to enhance information quality and operational efficiency.
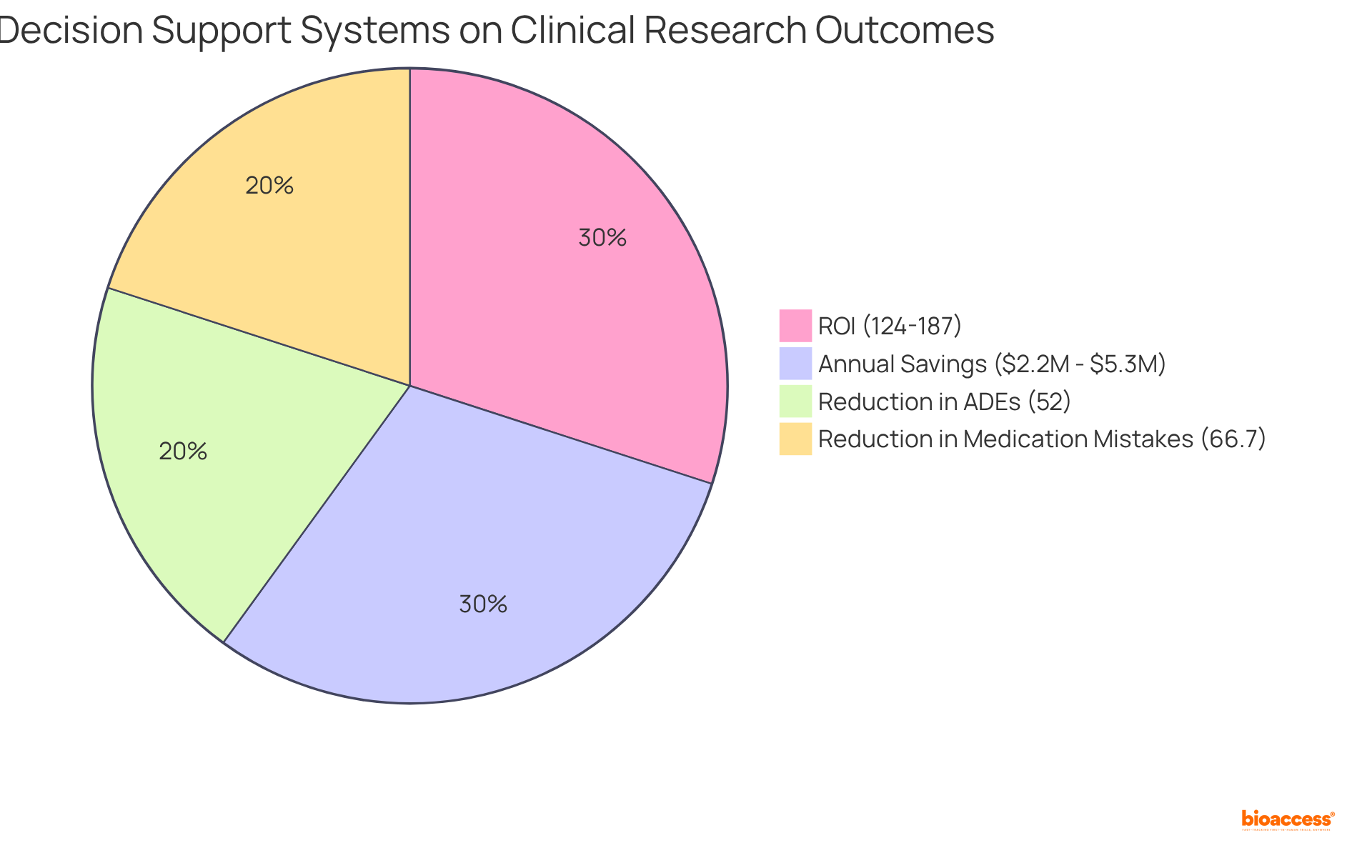
Decision Support Systems (DSS) play a crucial role in enhancing information analysis within medical research, significantly boosting the efficiency and effectiveness of trials. By providing real-time insights and advanced analytical capabilities, DSS empower researchers to make informed decisions swiftly. These systems enable the integration of data from diverse sources, facilitating comprehensive analyses that lead to improved research outcomes.
For instance, hospitals that have implemented advanced DSS report a return on investment (ROI) ranging from 124% to 187% within three years, translating to annual savings of $2.2 million to $5.3 million from prevented Adverse Drug Events (ADEs). Furthermore, research indicates that DSS can reduce medication mistakes by as much as 66.7% per 100 admissions, and the implementation of Clinical Decision Support (CDS) systems has been linked to a 52% reduction in ADEs in hospital environments, showcasing their potential to enhance patient safety and optimize workflows.
As the healthcare landscape continues to evolve, establishing governance structures for effective CDS implementation will be paramount. The integration of clinical data management systems will remain pivotal in transforming the efficiency of clinical research.
The landscape of clinical research is being profoundly reshaped by innovative clinical data management systems that enhance efficiency and compliance. By integrating advanced technologies such as artificial intelligence, decision support systems, and natural language processing, these systems not only streamline data collection and analysis but also ensure the integrity and reliability of research outcomes. Organizations that adopt these cutting-edge solutions are positioned to accelerate their research timelines, improve patient enrollment rates, and ultimately drive better health outcomes.
This article highlights several key players in this domain, including:
Each offering unique features that cater to the diverse needs of clinical studies. From bioaccess®'s rapid ethical approvals to Medidata Rave EDC's real-time data access, the capabilities of these systems are designed to tackle the persistent challenges faced by researchers today. Furthermore, the emphasis on training and skill development within organizations underscores the necessity of equipping teams with the tools and knowledge to navigate the complexities of modern clinical trials effectively.
As the demand for efficient clinical data management systems continues to grow, stakeholders must recognize the critical role these technologies play in advancing medical research. Embracing these innovations not only enhances operational workflows but also fosters a culture of compliance and quality assurance. The future of clinical research hinges on the ability to adapt and leverage these systems, ultimately ensuring that groundbreaking treatments reach patients more swiftly and safely.
What is bioaccess® and how does it enhance clinical research?
bioaccess® provides comprehensive information management solutions that improve the efficiency of research processes by securing ethical approvals in 4-6 weeks and increasing patient enrollment by 50% compared to traditional markets.
What industries benefit from bioaccess® services?
Medtech, Biopharma, and Radiopharma innovators are the primary industries that benefit from bioaccess® services, as it helps streamline their research studies.
How does bioaccess® address the challenges in clinical research?
bioaccess® implements advanced data management systems and integrates AI and analytics to expedite medical studies while ensuring reliability and compliance.
What is Medidata Rave EDC and what are its key features?
Medidata Rave EDC is an electronic data capture system designed to enhance clinical trial efficiency, featuring real-time information access and sophisticated reporting tools for informed decision-making.
How does the use of Medidata Rave EDC impact research?
The use of Medidata Rave EDC can improve data quality and reduce costs, with studies showing that approximately 41% were using an EDC system as of 2007.
What challenges do researchers face when adopting clinical data management systems?
Researchers face challenges such as high costs and insufficient technical support when adopting clinical data management systems.
What does LabKey offer for clinical data management?
LabKey provides a flexible platform for managing research information, featuring customizable tools for complex data handling and powerful analytics for improved decision-making.
Why is collaboration important in the Medtech sector according to LabKey?
Collaboration is essential in the Medtech sector to navigate the challenges of managing complex information and to enhance the overall quality of medical research.