


The essential features of a Clinical Trial Management System (CTMS) for clinical trials are critical for enhancing the efficiency of clinical studies. These features include:
Each element plays a significant role in streamlining management processes. Notably, these capabilities lead to:
Ultimately, this results in successful trial outcomes and ensures regulatory compliance, underscoring the importance of an effective CTMS in the clinical research landscape.
The landscape of clinical trials is evolving rapidly, driven by the pressing need for efficiency, accuracy, and compliance. Research teams are under increasing pressure to accelerate study timelines while upholding the highest standards. In this context, the implementation of a Clinical Trial Management System (CTMS) becomes paramount. This article explores the essential features of the bioaccess® CTMS, emphasizing how its innovative functionalities not only streamline operations but also enhance collaboration among stakeholders. Given the multitude of systems available, organizations must consider how to leverage the right tools to maximize their clinical research outcomes.
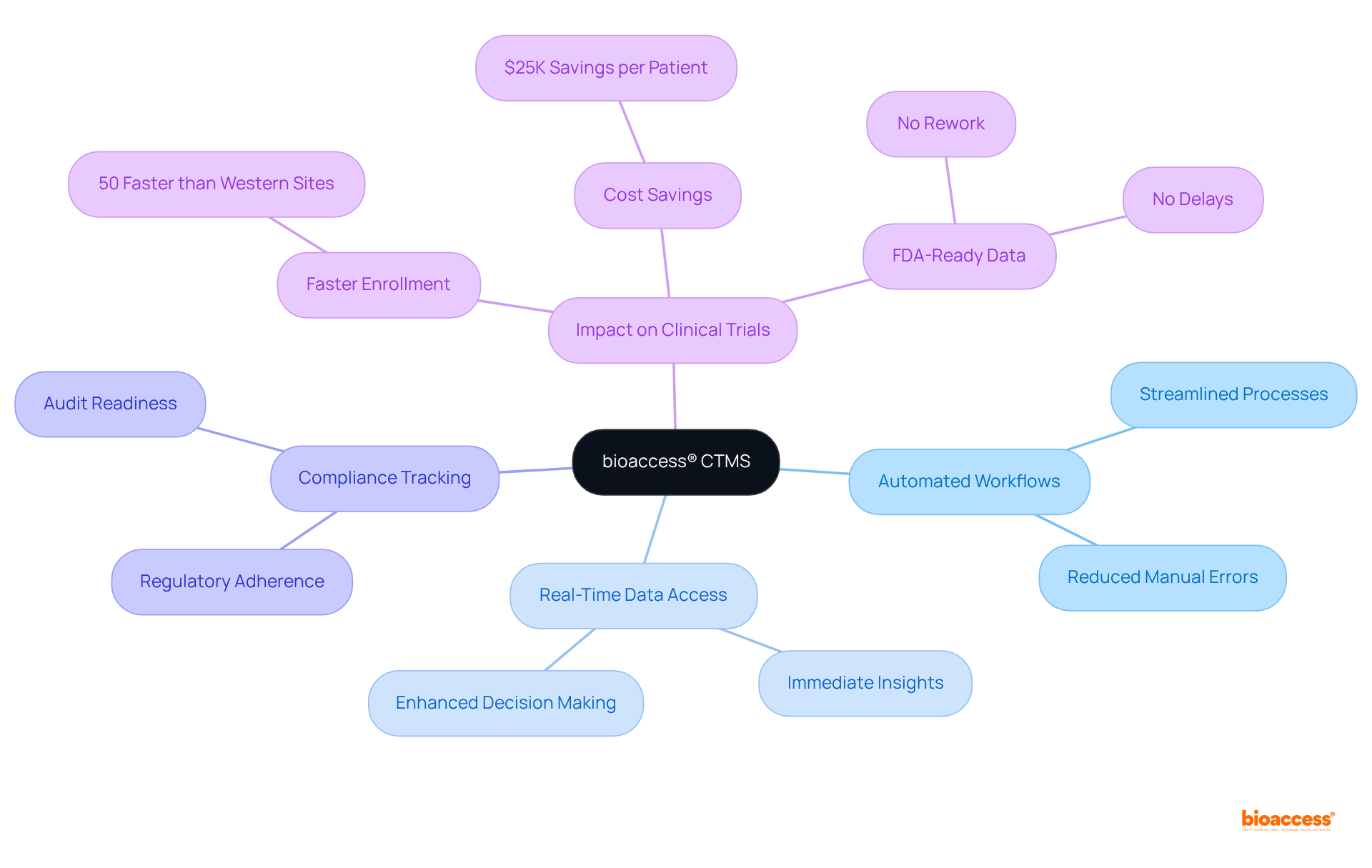
The ctms system, bioaccess® CTMS, is engineered to revolutionize research by integrating essential features that enhance both efficiency and compliance. With a concentrated focus on early-phase studies, including Early-Feasibility and First-In-Human studies, it significantly accelerates the approval process and participant enrollment. This ensures that clinical trials can advance swiftly and effectively.
The ctms system encompasses key functionalities such as:
Collectively, these features foster a more agile research environment. Notably, bioaccess® boasts over 20 years of experience in Medtech, enabling treatment-naive cardiology and neurology cohorts to be enrolled 50% faster than Western sites. This achievement translates into substantial cost savings of $25K per patient, all while providing FDA-ready data—no rework, no delays.
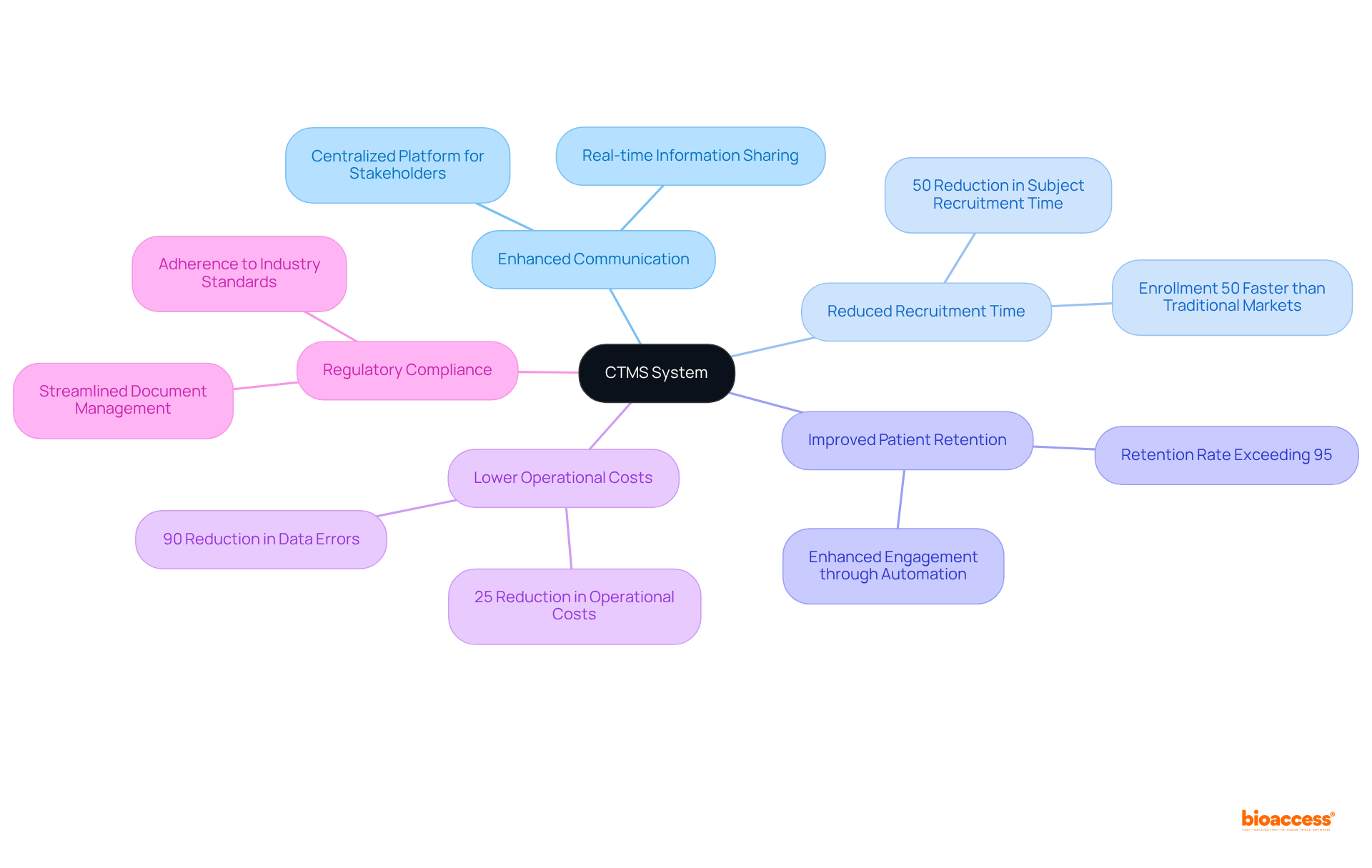
A robust ctms system significantly enhances stakeholder coordination by offering a centralized platform for communication and document sharing. This functionality facilitates seamless communication among research teams, sponsors, and regulatory bodies, ensuring alignment and clarity throughout the clinical research process. By fostering collaboration, the ctms system minimizes misunderstandings and accelerates decision-making, which is essential for achieving successful study outcomes.
Notably, GlobalCare Clinical Studies, in partnership with bioaccess™, achieved more than a 50% reduction in subject recruitment time and a retention rate exceeding 95% through the effective utilization of the ctms system in their ambulatory services in Colombia. Research indicates that the ctms system can enhance patient recruitment by 40%, reduce data errors by 90%, shorten study durations by 33%, and lower operational costs by 25%, underscoring its substantial impact on efficiency.
Furthermore, the implementation of the ctms system at institutions such as the Medical University of South Carolina resulted in improved patient recruitment and financial outcomes, demonstrating how centralized communication platforms can transform research management. Additionally, bioaccess™ offers comprehensive study management services, including feasibility assessments and compliance evaluations, which further enhance the effectiveness of the ctms system.
The collaboration between IDx Technologies and bioaccess™ for data licensing in Latin America exemplifies the role of CTMS in advancing AI-driven disease detection in ophthalmology. The ctms system enables prompt information gathering and participant advancement monitoring, which is crucial for maintaining study schedules and ensuring adherence to regulatory standards.
Ultimately, the integration of the ctms system not only streamlines operations but also fosters a collaborative environment that is essential for advancing clinical research and positively impacting local economies.
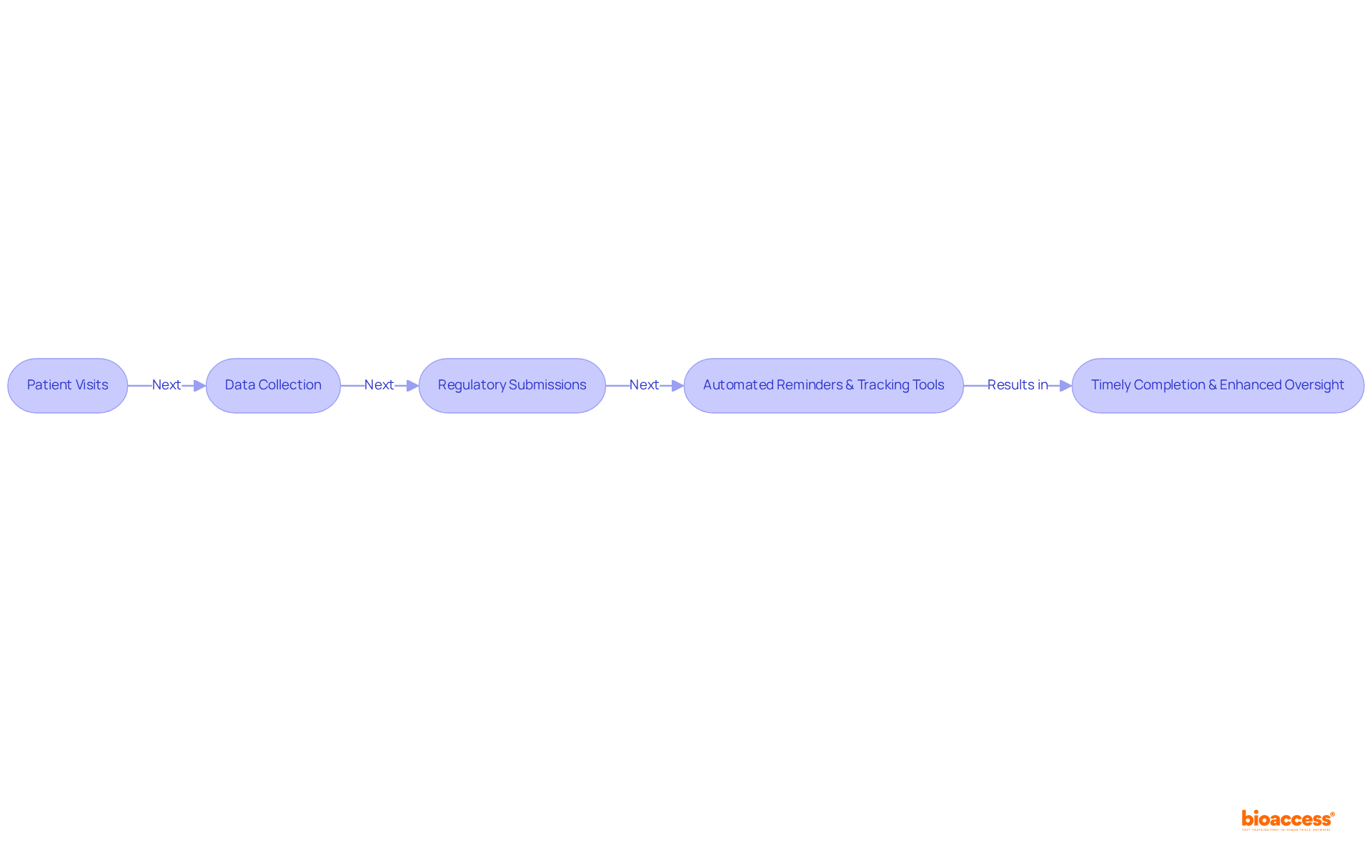
The scheduling features of the ctms system facilitate a meticulous organization of study activities, which include:
By utilizing automated reminders and tracking tools, the ctms system ensures the timely completion of all tasks. This enhanced oversight not only strengthens accountability but also allows for the early detection of potential delays, enabling prompt interventions. Notably, the ctms system accelerates patient enrollment for cardiology and neurology groups by 50% compared to Western locations, achieving significant cost savings of $25K per patient with FDA-ready data—no rework, no delays. With over 15 years of experience in the sector, the company affirms that the ctms system is vital in maintaining the integrity of clinical study schedules, ultimately leading to more favorable outcomes.
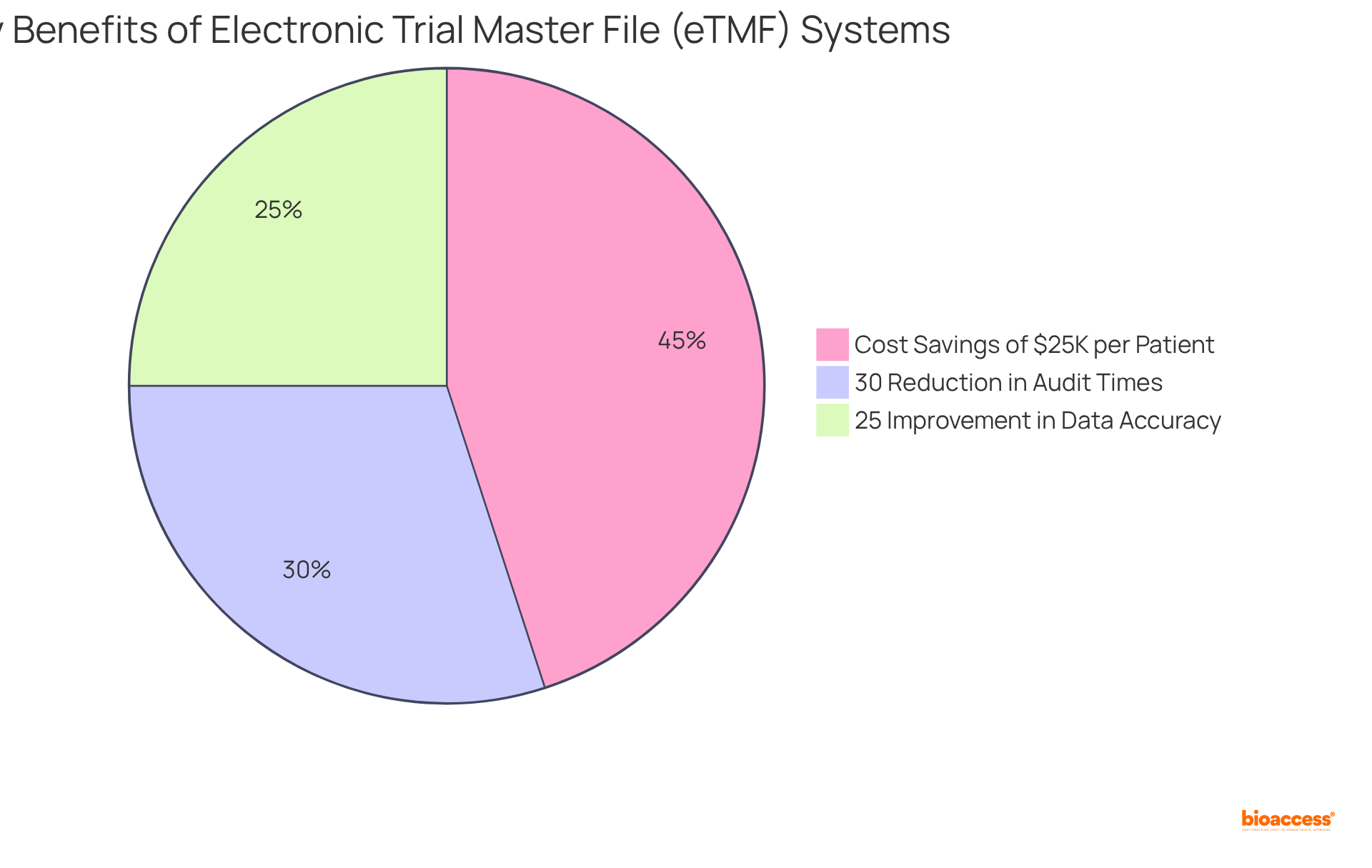
bioaccess®'s Clinical Study Management ctms system incorporates an electronic Trial Master File (eTMF), centralizing all study documentation for secure storage and easy access to records. This functionality is crucial for compliance with regulatory standards, as it offers comprehensive audit trails and robust version control, essential for demonstrating adherence to protocols. By prioritizing information integrity, the eTMF system significantly enhances the credibility of trial outcomes.
Organizations utilizing eTMF systems have reported:
This underscores the effectiveness of these tools in maintaining compliance and ensuring high-quality data management. Furthermore, the eTMF's real-time monitoring capabilities facilitate proactive compliance management, enabling clinical teams to swiftly address any discrepancies and uphold the integrity of their research.
For instance, a leading pharmaceutical company experienced a 30% reduction in trial timelines after adopting an eTMF, illustrating how these systems can streamline operations and enhance regulatory compliance. As the service provides ethical approvals in 4-6 weeks and achieves enrollment 50% faster than conventional markets, the incorporation of the ctms system becomes even more essential in supporting these swift advancements.
By leveraging the expertise of the company, organizations can not only improve their compliance efforts but also realize substantial cost savings of $25K per patient with FDA-ready data, further maximizing the advantages of eTMF systems. To capitalize on these benefits, organizations should regularly evaluate their implementation processes and ensure alignment with their specific compliance needs.
The ctms system from a leading provider stands out with robust milestone planning tools that empower project managers to establish clear objectives and deadlines for each phase of a study. By efficiently allocating tasks to team members and monitoring their progress, the ctms system provides real-time insights into project advancement. This proactive approach not only identifies potential bottlenecks in the ctms system but also facilitates timely resource reallocation, ensuring that evaluations remain on schedule.
Notably, with expertise in managing Early-Feasibility, First-In-Human, Pilot, Pivotal, and Post-Market Follow-Up Studies in Latin America, clinical studies can achieve patient enrollment 50% faster than in Western locations. This efficiency translates to substantial cost reductions of $25K per patient with FDA-ready data—eliminating rework and delays. Such support simplifies setup and compliance evaluations while effectively addressing the recruitment challenges faced by Medtech and Biopharma startups.
The ctms system, bioaccess® CTMS, is purposefully designed to manage financial agreements and participant remuneration with precision, ensuring adherence to budgets while upholding ethical standards. The ctms system offers robust monitoring of expenses and payments, fostering transparency and accountability throughout the financial process. By streamlining these operations through the ctms system, it alleviates administrative burdens, thereby enhancing relationships with participants and stakeholders.
Ethical compensation is paramount in medical research; it not only honors participants' contributions but also cultivates trust and engagement. As we approach 2025, the emphasis on equitable pay practices becomes increasingly significant, with studies revealing that well-structured compensation can markedly reduce participant dropout rates and enhance overall efficiency.
For example, the implementation of clear financial agreements and transparent communication regarding compensation can lead to improved participant retention and satisfaction. By leveraging historical data analysis on participant compensation practices, the company ensures that its financial management strategies align with industry standards, which ultimately enhances the ctms system and contributes to the success of research studies.
Furthermore, addressing financial challenges such as budget overages and unchecked spending is crucial for maintaining trial integrity. Negotiating better contracts with sponsors further strengthens financial management, ensuring alignment among all parties regarding budget expectations.
Integrating participant monitoring tools with Electronic Information Capture (EDC) systems in the ctms system bioaccess® significantly enhances information accuracy in clinical research. This synchronization not only facilitates real-time updates on participant status and information gathering but also effectively reduces discrepancies. By ensuring precise record-keeping, the system bolsters the reliability of test outcomes while strengthening adherence to regulatory standards.
Research has demonstrated that efficient EDC synchronization can reduce entry errors by over 30%, thereby enhancing overall information integrity. As we approach 2025, the importance of such synchronization becomes increasingly clear, with regulatory agencies placing greater emphasis on data accuracy and transparency in research studies.
Furthermore, the bioaccess® ctms system offers comprehensive clinical study management services, including:
Collectively, these services enhance the success of clinical studies and their positive impact on local economies.
The ctms system automates the creation of inspection reports and correspondence, significantly enhancing communication with regulatory bodies and stakeholders. This feature alleviates the administrative burden on teams, allowing them to focus on critical activities. By ensuring timely and accurate communication, the ctms system boosts overall trial management efficiency and supports compliance efforts.
Furthermore, the company provides comprehensive services, including:
These services are essential for expediting regulatory approvals. Notably, this product secures regulatory approvals in just 6-8 weeks, compared to the typical 6-12 months in the US/EU, underscoring the importance of efficient regulatory communication. As the clinical research landscape evolves in 2025, the ctms system's capability to automate correspondence will be vital for maintaining compliance and accelerating approval processes.
The ctms system from bioaccess® is equipped with advanced analytics and business intelligence tools that significantly enhance study performance. With over 20 years of experience in Medtech, our team meticulously analyzes data trends and outcomes, providing researchers with crucial insights that inform strategic decisions, thereby improving study efficiency and effectiveness. This analytical prowess facilitates proactive adjustments to research protocols and resource allocation, fostering better patient outcomes and ensuring the successful completion of studies.
Moreover, its flexible approach to managing research studies through the ctms system allows for tailored methodologies that meet specific study needs. By leveraging FDA-prepared information, studies can achieve patient enrollment 50% faster than in Western locations, resulting in substantial cost savings of $25K per individual. This innovative approach, utilizing the ctms system, ensures that studies are not only efficient but also compliant with regulatory standards, positioning the organization as a frontrunner in Medtech clinical research across Latin America.
Utilizing a CTMS, such as bioaccess®, offers significant advantages that are crucial in the realm of clinical research. These include:
By centralizing management activities, the system reduces administrative burdens and fosters collaboration among stakeholders. Organizations that have adopted CTMS have reported decreases in study start-up times by as much as 30%, a testament to its efficiency in expediting processes. Furthermore, the data analytics capabilities empower research directors to make informed decisions, optimizing study strategies and enhancing data integrity. This aspect is particularly vital, as centralized monitoring can identify operational risks early, facilitating timely interventions that safeguard trial integrity. Overall, these insights not only contribute to successful outcomes but also expedite product development, positioning organizations at the forefront of innovation in clinical research.
The bioaccess® CTMS system stands as a transformative tool, fundamentally designed to enhance the efficiency and compliance of clinical trials. By integrating essential features that streamline processes, facilitate stakeholder collaboration, and ensure data integrity, this system significantly accelerates research timelines and improves overall study outcomes. The emphasis on early-phase studies and the ability to deliver FDA-ready data without delays underscores the critical role that CTMS plays in advancing clinical research.
Key functionalities such as automated workflows, real-time data access, and comprehensive financial management not only optimize trial management but also foster a collaborative environment among all stakeholders involved. The impressive statistics highlighting faster patient enrollment, reduced operational costs, and improved compliance demonstrate the tangible benefits of utilizing a CTMS in clinical research. Institutions leveraging these systems have reported remarkable improvements in study efficiency and participant retention, reinforcing the value of adopting such innovative solutions.
As the landscape of clinical research continues to evolve, the importance of utilizing a robust CTMS cannot be overstated. Organizations seeking to remain competitive must prioritize the implementation of systems that enhance operational efficiency and ensure compliance with regulatory standards. By embracing the advanced features of bioaccess® CTMS, research teams can not only improve their study outcomes but also contribute to the advancement of medical science, ultimately benefiting patients and communities alike.
What is bioaccess® CTMS?
bioaccess® CTMS is a clinical trial management system designed to enhance efficiency and compliance in clinical research, particularly focusing on early-phase studies like Early-Feasibility and First-In-Human studies.
What are the key features of the bioaccess® CTMS?
The key features of bioaccess® CTMS include automated workflows, real-time data access, and compliance tracking, all of which contribute to a more agile research environment.
How does bioaccess® CTMS improve participant enrollment and cost savings?
The system enables faster enrollment of treatment-naive cardiology and neurology cohorts, achieving enrollment rates 50% faster than Western sites and resulting in cost savings of $25K per patient while providing FDA-ready data.
How does the ctms system enhance stakeholder coordination in clinical trials?
The ctms system offers a centralized platform for communication and document sharing, facilitating seamless collaboration among research teams, sponsors, and regulatory bodies, which minimizes misunderstandings and accelerates decision-making.
What impact has the ctms system had on patient recruitment and study durations?
The ctms system can enhance patient recruitment by 40%, reduce data errors by 90%, shorten study durations by 33%, and lower operational costs by 25%.
How does the scheduling feature of the ctms system streamline trial management?
The scheduling features help organize study activities such as patient visits, data collection, and regulatory submissions, using automated reminders and tracking tools to ensure timely task completion and early detection of potential delays.
What are the benefits of implementing the ctms system at research institutions?
Implementation of the ctms system has led to improved patient recruitment and financial outcomes, demonstrating its effectiveness in transforming research management.
Can you provide an example of a successful collaboration using the ctms system?
A collaboration between IDx Technologies and bioaccess™ for data licensing in Latin America illustrates the ctms system's role in advancing AI-driven disease detection in ophthalmology, enabling efficient information gathering and participant monitoring.
How does the ctms system contribute to local economies?
By streamlining operations and fostering a collaborative environment in clinical research, the ctms system positively impacts local economies through improved research outcomes and efficiency.