

The article emphasizes crucial insights that innovators must grasp regarding EU Medical Device Regulation, spotlighting compliance strategies and the regulatory landscape. It asserts the significance of navigating the stringent requirements for safety and performance, while also advocating for the utilization of various regulatory pathways. These pathways can expedite market access and ensure adherence to the evolving standards that govern the industry.
Navigating the complexities of the EU Medical Device Regulation presents a significant challenge for innovators aiming to introduce their healthcare products to the market. This comprehensive framework establishes stringent safety and performance standards, while simultaneously offering a landscape abundant with opportunities for those adept at maneuvering through its requirements. As the regulatory environment evolves, particularly with updates anticipated in 2025, manufacturers must consider how to ensure compliance while expediting their market entry. Grasping these essential insights empowers innovators to thrive amid the challenges posed by the EU medical device landscape.
bioaccess® effectively leverages Colombia's regulatory speed and cost efficiency, alongside the Balkans' diverse patient populations and Australia's streamlined pathways, to facilitate rapid ethical approvals and expedite enrollment for clinical trials.
Colombia presents significant advantages, including a remarkable 30% cost savings compared to North America and Western Europe, with IRB/EC and MoH (INVIMA) reviews completed in a swift 90-120 days.
Moreover, Colombia's healthcare system is highly ranked globally, further enhancing its appeal for patient recruitment. This distinctive approach empowers Medtech and Biopharma innovators to bring their products to market more efficiently, significantly reducing the time and cost associated with clinical research under the EU medical device regulation.
Furthermore, bioaccess®'s collaboration with Caribbean Health Group is strategically positioned to establish Barranquilla as a premier destination for clinical trials in Latin America, supported by the Colombian Minister of Health, which enhances patient recruitment and retention rates in the region.
The EU Medical Device Regulation establishes stringent requirements for the safety and performance of healthcare products, underscoring its significance in the clinical research landscape. This EU medical device regulation not only substitutes prior guidelines but also aims to enhance patient safety and ensure the provision of high-quality healthcare instruments. Innovators must adeptly navigate this comprehensive framework, which encompasses essential requirements, clinical assessments, and post-market surveillance. Successfully promoting their products in the EU hinges on their ability to comply with these rigorous standards.
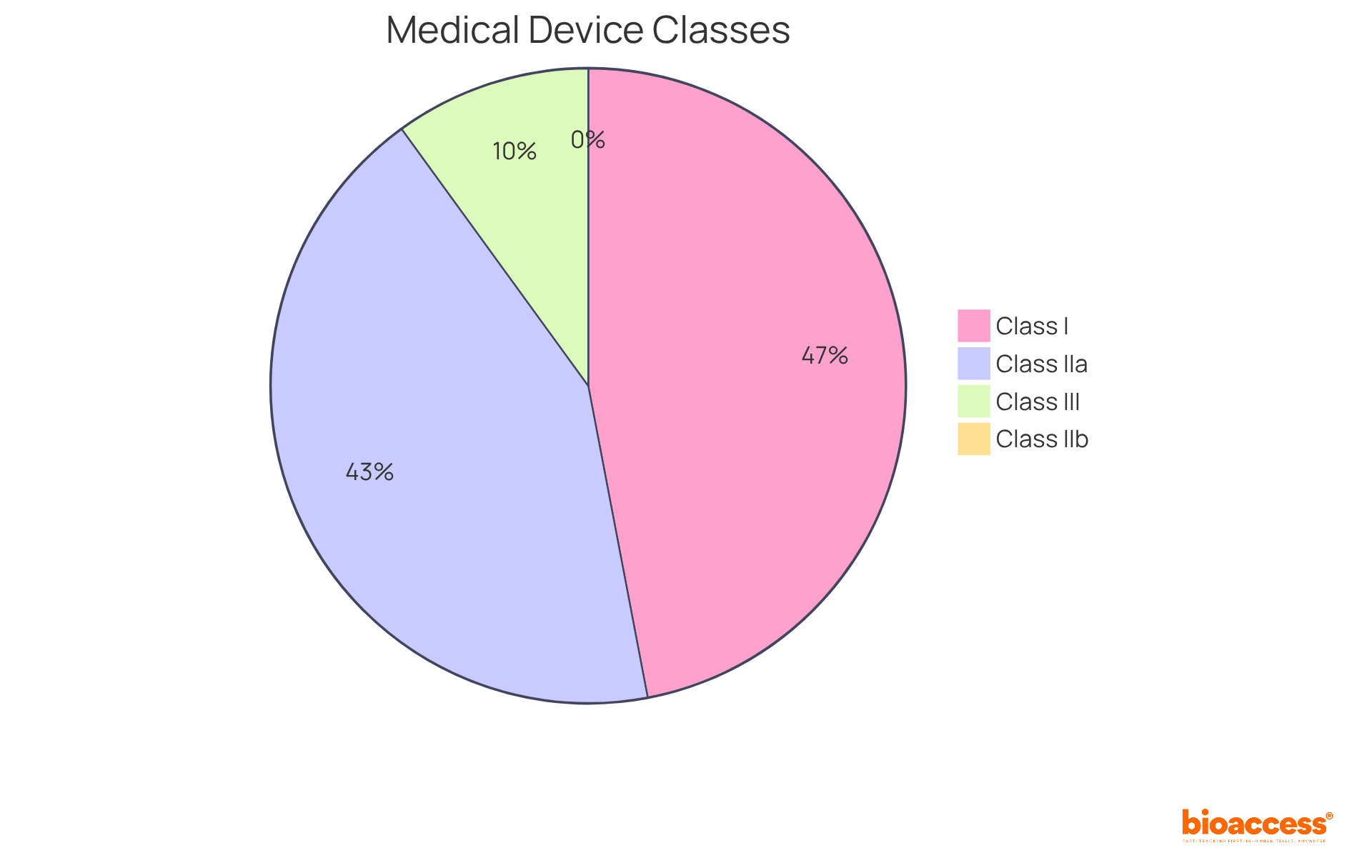
In the EU, healthcare instruments are categorized into four primary classes:
Each classification entails distinct compliance requirements, with higher classes necessitating more extensive clinical evaluations and oversight. Notably, 47% of medical products are classified as Class I, where 95% are exempt from extensive regulatory processes. This exemption allows manufacturers to market these items without prior FDA clearance, provided they register their establishment and list their products. Conversely, Class III products, representing approximately 10% of the total, undergo rigorous examination due to their high-risk nature, necessitating thorough adherence strategies to ensure safety and effectiveness. Understanding these classifications is essential for innovators, as it directly impacts their compliance pathways and market entry strategies under the EU medical device regulation for 2025 and beyond.
To obtain CE marking, manufacturers must demonstrate that their medical products adhere to the EU medical device regulation safety and performance standards. This necessitates assembling a thorough technical dossier, conducting essential clinical assessments, and collaborating with a Notified Body, particularly for higher-risk products.
The CE marking process is intricate, with evolving requirements for 2025 that include enhanced documentation and post-market surveillance obligations in accordance with the EU medical device regulation. Statistics reveal that approximately 90% of medium-risk products are evaluated based on substantial equivalence, often without new clinical data, highlighting the critical importance of meticulous technical file preparation.
Industry leaders emphasize that challenges in acquiring CE marking can stem from varying interpretations of the EU medical device regulation among EU member states, making it essential for manufacturers to remain informed and proactive in their adherence strategies.
Notified Bodies are pivotal organizations designated by EU member countries to evaluate the compliance of healthcare products prior to market access. Their responsibilities encompass assessing technical documentation, conducting thorough audits, and ensuring adherence to the EU medical device regulation 2017/745 (MDR). Recent updates in 2025 underscore the critical role these organizations play in maintaining high standards of safety and effectiveness in medical instruments.
Engaging with a Notified Body early in the development process is vital for innovators. This proactive approach can significantly streamline the approval process, reducing the risk of delays stemming from regulatory challenges. Statistics indicate that the effectiveness of audits conducted by Notified Bodies often hinges on the quality of the technical file provided, which serves as a comprehensive evidence package demonstrating safety and compliance.
Regulatory experts, such as Ana Criado, Director of Regulatory Affairs and CEO of Mahu Pharma, and Katherine Ruiz, an expert in Regulatory Affairs for Medical Devices and In Vitro Diagnostics in Colombia, stress the importance of viewing interactions with Notified Bodies as collaborative partnerships. Ana's extensive experience in regulatory affairs, combined with Katherine's expertise in medical equipment regulations, highlights the value of early engagement, which fosters alignment with regulatory expectations and can lead to more favorable outcomes during audits. A well-prepared technical file is indispensable; without it, the risk of jeopardizing CE marking approval escalates.
Moreover, the focus on data-driven, risk-based Post-Market Surveillance (PMS) systems has emerged as a central theme in the context of EU medical device regulation during compliance audits. Notified Bodies are now tasked with evaluating not only the documentation but also the efficacy of PMS strategies in ensuring ongoing safety throughout the product's lifecycle. As noted, "They seek data-informed, risk-oriented PMS systems that demonstrate that healthcare products remain secure and efficient throughout their lifecycle." By understanding these dynamics and leveraging the insights of experts like Ana and Katherine, manufacturers can enhance their adherence strategies and facilitate smoother market entry for their healthcare products.
To prepare effectively for an audit with a Notified Body, manufacturers should:
Clinical evaluation serves as a systematic process designed to assess clinical data, confirming the safety and performance of medical instruments. This evaluation is vital for compliance with the EU medical device regulation, which requires manufacturers to provide robust evidence of their products' efficacy and safety. Equally essential is post-market surveillance (PMS), which entails ongoing monitoring of a product's performance once it has entered the market. This process is crucial for swiftly identifying and addressing any emerging issues, ultimately safeguarding patient safety.
Statistics reveal that a significant number of post-market incidents are reported within the EU, emphasizing the necessity for effective PMS strategies. For example, proactive PMS can prevent adverse events and enhance product development, as demonstrated by companies that incorporate PMS into their quality management systems. Effective strategies encompass:
With the forthcoming updates to the EU Medical Device Regulation in 2025, manufacturers must adapt their PMS practices to align with the new regulatory expectations. This adaptation includes establishing comprehensive post-market surveillance plans that comply with the latest guidelines. bioaccess® offers expedited clinical trial services in Latin America, drawing on over 20 years of experience in managing Early-Feasibility Studies (EFS), First-In-Human Studies (FIH), Pilot Studies, Pivotal Studies, and Post-Market Clinical Follow-Up Studies (PMCF). The emphasis on PMS reflects a broader acknowledgment of its significance in upholding regulations and ensuring the long-term safety and efficacy of healthcare products in the market.
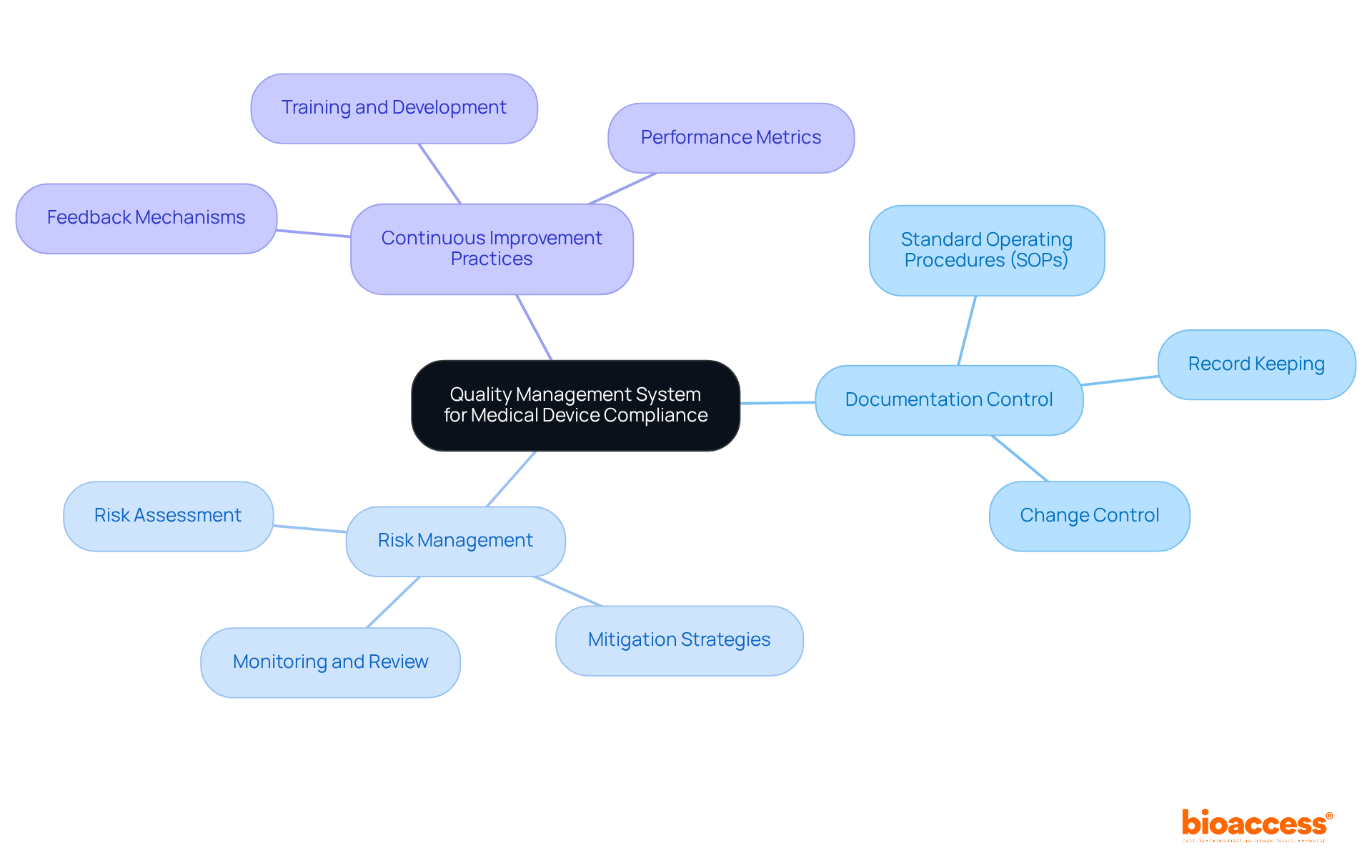
Establishing a Quality Management System (QMS) is crucial for manufacturers of healthcare products to ensure compliance with the Medical Device Regulation (MDR). A well-implemented QMS empowers organizations to effectively manage processes, enhance product quality, and adhere to regulatory requirements.
Essential components of a QMS include:
These elements are vital for upholding high standards throughout the product lifecycle, ultimately fostering trust and reliability in healthcare offerings.
Innovative healthcare instruments can significantly benefit from various regulatory pathways, particularly the Fast Track designation and the Innovative Instrument designation, both of which are designed to expedite the approval process. The Fast Track designation facilitates a streamlined review process, thereby reducing time to market considerably. Recent data reveals that products qualifying for these pathways can experience approval times that are up to 50% faster than traditional routes, thereby enhancing their competitiveness in a rapidly evolving market.
For example, in 2025, several medical devices received Fast Track designation, illustrating the effectiveness of this pathway in ensuring timely access to innovative solutions. Businesses leveraging the Fast Track designation report quicker market entry and improved collaboration with oversight organizations, which ensures compliance while fostering innovation.
Experts such as Ana Criado, Director of Regulatory Affairs, underscore the importance of understanding these compliance pathways. With her extensive expertise in health economics and compliance consulting, she asserts that strategic planning around these designations can yield substantial advantages in market timing and overall product success. Grasping these governance pathways is crucial for manufacturers aiming to strategically navigate their approach.
Brexit has led to the establishment of distinct regulatory frameworks for the UK and EU, significantly impacting the governance of healthcare instruments. Manufacturers are now required to navigate both the EU medical device regulation and the UK's Medical Device Regulations, which may present differing requirements and processes. Understanding these regulatory changes is crucial for ensuring compliance and maintaining market access in both regions.
Future trends in the EU medical device regulation are set to emphasize digital health technologies, artificial intelligence, and sustainability. This evolving regulatory landscape demands that manufacturers remain vigilant and informed about these developments. By adapting their compliance strategies, they can not only meet new requirements but also seize opportunities for innovation. Staying ahead of these trends is not merely advantageous; it is essential for thriving in a competitive Medtech environment.
The landscape of EU medical device regulation presents both challenges and opportunities for innovators. Understanding the intricacies of this regulatory framework is crucial for successfully navigating the complexities of compliance and market entry. By leveraging insights into clinical evaluations, CE marking processes, and the roles of Notified Bodies, manufacturers can better position themselves to meet stringent safety and performance standards while expediting their time to market.
Key insights discussed in this article highlight the importance of:
Additionally, recognizing the impact of Brexit on regulatory frameworks and staying informed about future trends, such as digital health technologies and sustainability, are vital for maintaining a competitive edge in the Medtech industry.
In conclusion, the journey through EU medical device regulation is multifaceted and requires a proactive approach. Innovators are encouraged to embrace strategic planning, engage with regulatory experts, and continually adapt to evolving requirements. By doing so, they not only ensure compliance but also foster innovation that can lead to significant advancements in healthcare solutions. Staying ahead of these regulatory trends is essential for thriving in a dynamic and competitive environment.
What is bioaccess® and how does it facilitate clinical research for medical devices in the EU?
bioaccess® leverages Colombia's regulatory speed and cost efficiency, the Balkans' diverse patient populations, and Australia's streamlined pathways to facilitate rapid ethical approvals and expedite enrollment for clinical trials.
What are the cost advantages of conducting clinical trials in Colombia?
Conducting clinical trials in Colombia can result in significant cost savings of about 30% compared to North America and Western Europe, with IRB/EC and MoH (INVIMA) reviews completed within 90-120 days.
How does Colombia's healthcare system contribute to clinical trials?
Colombia's healthcare system is highly ranked globally, enhancing its appeal for patient recruitment in clinical trials.
What is the significance of the EU Medical Device Regulation?
The EU Medical Device Regulation establishes stringent requirements for the safety and performance of healthcare products, aiming to enhance patient safety and ensure high-quality healthcare instruments.
What are the key categories of medical device classification in the EU?
Medical devices in the EU are classified into four primary classes: Class I (low risk), Class IIa (medium risk), Class IIb (higher risk), and Class III (highest risk).
What are the compliance requirements for different classes of medical devices?
Higher classification classes (e.g., Class IIb and Class III) necessitate more extensive clinical evaluations and oversight, while 95% of Class I products are exempt from extensive regulatory processes, allowing manufacturers to market them without prior FDA clearance.
Why is understanding medical device classifications important for innovators?
Understanding these classifications is essential for innovators, as it directly impacts their compliance pathways and market entry strategies under the EU medical device regulation for 2025 and beyond.